31 | Motion-Sparing Technology |
 | Case Presentation |
A 46-year-old male presented with a 3-month history of neck and right upper extremity discomfort. The patient stated that his pain originated in his neck and radiated down his right arm into his thumb and index finger. He also noted worsening fine motor coordination in his right hand but denied any gait imbalance or urinary dysfunction. The patient had already used nonsteroidal anti-inflammatory medications, cervical traction, and a transcutaneous electrical nerve stimulation (TENS) unit with little improvement in his symptoms. Physical examination findings included a positive Spurling sign, moderate weakness in his right wrist extensor muscles, and decreased sensation to light touch in his right thumb and index finger. The patient had normal deep tendon reflexes bilaterally and no pathological long tract signs. Plain radiographs of the cervical spine demonstrated spondylotic changes involving the C5-6 level with no evidence of instability on dynamic films (Fig. 31–1). Magnetic resonance imaging (MRI) of the cervical spine revealed a right paramedian herniated nucleus pulposus that impinged upon the exiting right C6 nerve root with no significant compression of the spinal cord (Fig. 31–2A,B). Because his symptoms had progressed despite multiple conservative treatments, including a right C6 transforaminal epidural steroid injection, the patient elected to undergo cervical disk replacement at the C5-6 level (Fig. 31–3A,B).
 | Background |
Degenerative changes of the cervical spine represent a nearly ubiquitous radiographic finding in older individuals, and as our population continues to age it is expected that the incidence of cervical spondylosis will increase as well.1–4 Since its introduction in the 1950s, first by Robinson and Smith5 and then by Cloward,6 anterior cervical diskectomy and fusion (ACDF) has become the gold standard treatment for symptomatic myelopathy and radiculopathy secondary to cervical spondylosis.1,7–9 Unfortunately this surgical technique is associated with several complications. The harvesting of autogenous iliac crest bone graft may result in significant morbidity, including intractable pain, wound infections, and hematoma formation, whereas the use of allograft carries an inherent risk of disease transmission to the recipient.10 Pseudarthrosis may occur in up to 20% of cervical fusions, which may lead to persistent pain, progressive deformity, and neurological deficits.11 Consequently, in an attempt to prevent the development of a pseudarthrosis, most surgeons recommend some type of postoperative immobilization after ACDF.12–16
Because this surgical technique converts a formerly mobile triple joint complex into a fixed unit, it has been suggested that arthrodesis of the cervical spine may subject the adjacent unfused levels to greater biomechanical stresses and hasten the degeneration of these motion segments.1,17–20 Although there is some evidence to suggest that adjacent level disease may simply represent the natural progression of patients who are inherently predisposed to acquiring cervical spondylosis,21 several other studies appear to establish a relationship between cervical fusion and the degeneration of adjacent spinal segments. In various retrospective reviews, the rate of adjacent segment degeneration following cervical fusion has been reported to be as high as 92%.21–27 Hilibrand et al published the long-term results of 374 consecutive patients who underwent ACDF for spondylotic radiculopathy or myelopathy.26 Over a 2- to 21-year follow-up period, the overall prevalence of symptomatic adjacent level disease requiring surgery was 14.2% with an annual incidence of 2.9%. In response to these concerns regarding postarthrodesis adjacent segment degeneration, motion-sparing technologies such as cervical disk arthroplasty have been proposed as potential alternatives to cervical fusion. By retaining the mobility of the affected spinal segment requiring surgical treatment, it is thought that these nonfusion techniques may reduce the biomechanical loads on adjacent segments and limit their subsequent degeneration.
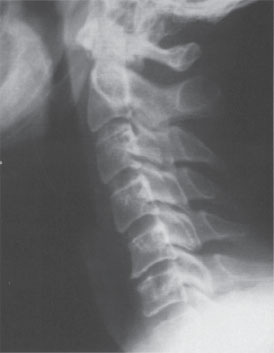
Figure 31–1 Preoperative lateral radiograph.
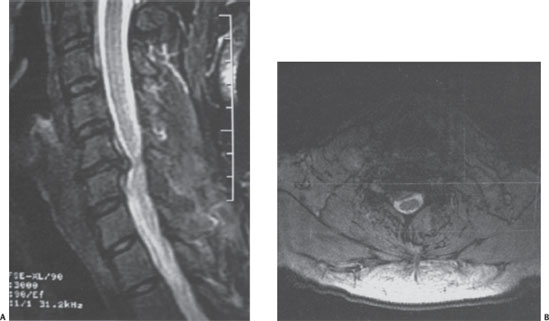
Figure 31–2 (A) T2-weighted sagittal magnetic resonance imaging (MRI) demonstrates a herniated nucleus pulposus at the C5-6 level. (B) T2-weighted axial MRI reveals impingement of the right C6 nerve root with no significant compression of the spinal cord.
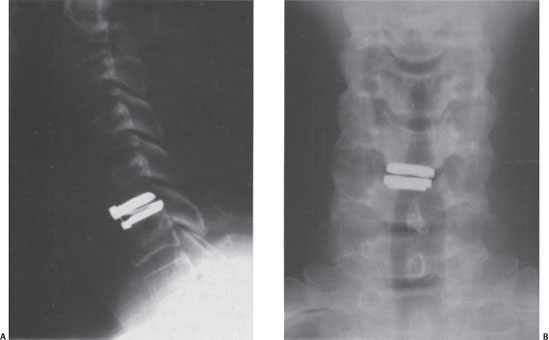
Figure 31–3 (A) Postoperative lateral radiograph following implantation of a cervical disk replacement at the C5-6 level. (B) Postoperative anteroposterior radiograph.
Unlike ACDF procedures, which have been shown to alter the kinematics at adjacent levels,18,28 multiple in vivo and in vitro biomechanical studies have demonstrated that cervical disk replacements may more closely approximate the physiological motion of the cervical spine and may therefore slow the progression of adjacent segment degeneration.1,18,28–33 Wigfield et al demonstrated that the stress profiles of the intervertebral disks adjacent to a cervical arthroplasty implant were similar to those recorded in untreated, intact specimens.19 Other cadaveric studies have also confirmed that these artificial disks may reproduce the normal biomechanics of both the operated and adjacent spinal segments.32,34 Pickett et al analyzed the kinematics of 20 patients who underwent cervical disk replacement using the Bryan prosthesis at one or two levels for cervical spondylosis. At 2-year follow-up, the authors did not observe any significant differences in the center of rotation or range of motion compared with preoperative values.33
 | Design Considerations |
With any prosthesis it is imperative to understand its specific design characteristics to formulate appropriate surgical techniques for its insertion, predict its long-term function, and anticipate potential complications related to its use. Principal design considerations for cervical disk replacement include the kinematics and biomaterials of the articulation, implant dimensions, footplate geometry, and method of fixation.1,35,36 According to Phillips and Garfin, the ideal cervical disk replacement would tolerate physiological loads without premature fatigue or failure, exhibit superior wear properties while generating minimal debris particles, and be easily secured to the surrounding vertebral bodies.1 In addition, to protect the facet joints from abnormal biomechanical stresses, a cervical implant should also have an axis of rotation that is similar to that of the normal spine.
There are primarily three classes of articulations that are utilized for cervical disk arthroplasty: constrained, semiconstrained, and unconstrained. A device is considered to be constrained if it includes a mechanical stop within the physiological range of motion, whereas with a semiconstrained prosthesis the mechanical stop is outside the normal range of motion; if an implant has no mechanical stop at all it is classified as unconstrained. In addition, current cervical disk replacements may either employ a ball and socket articulation that generates purely rotational motion or a saddle type of joint that allows for both rotation as well as some translation.1
Constrained prostheses generally exhibit greater stability, and their fixed axis of rotation serves to minimize the sheer forces on the facet joints.1,35 Unfortunately, these constrained devices place greater stresses on the implant-bone interfaces and are technically less forgiving, requiring more precise placement to effectively reproduce the natural axis of rotation of the cervical spine.1,18,35,37 In contrast, because unconstrained implants allow some degree of translation, there is decreased stress concentration at specific points on the articulating surface and they appear to be more forgiving in terms of their placement in the coronal and sagittal planes.1,35 However, this lack of constraint provides less stability to the motion segment and exposes the adjacent facet joints to greater sheer and torsional loads.1,37–39 As a result, maintaining proper soft tissue tension is of the utmost importance for unconstrained devices; McAfee et al demonstrated that the posterior longitudinal ligament plays an essential role in enhancingstability following diskectomy for cervical disk replacements.40
As with total joint arthroplasty of the extremities, the material properties of cervical disk replacements are a significant consideration. Stainless steel has not been implemented for cervical arthroplasty because of its predilection for corrosion and its susceptibility to fatigue fracture. The end plates as well as some of the articulations of most cervical prostheses currently in development are composed of either cobalt-chromium or titanium, both of which have been used extensively for total joint arthroplasty because of their biocompatibility, resistance to corrosion, and superior biomechanical properties.1,35,38,41–43 However, one of the major advantages of titanium implants over cobalt-chromium is its MRI compatibility, which obviously facilitates any postoperative imaging of the spinal segment.
Another factor that will be critical in determining the success of cervical disk replacement is implant stability. Long-term implant fixation is dependent upon solid bony ingrowth into the press-fit surfaces of the prosthesis.1,35,40 For bone formation to occur, the implant must be stable and the end plates must have adequate pore size and geometry.1,35 To facilitate this process, several surface coatings have been developed, including plasma-sprayed titanium, titanium wire mesh, aluminum oxide, porous cobalt-chromium, and bioactive materials such as hydroxyapatite and calcium phosphate. Many of the original cervical disk replacements required supplementary screw fixation into the vertebral bodies to augment stability, but this type of fixation has largely been abandoned in favor of keels or spikes located on the base plates of newer devices.
 | Indications and Contraindications |
At this time the definitive indications for cervical arthroplasty remain a matter of some debate. This technique is typically reserved for individuals with symptomatic radiculopathy secondary to a herniated nucleus pulposus or spondylosis who have failed conservative management and would otherwise undergo ACDF.1,44–48 Although cervical disk replacements have already been used to treat multilevel spondylotic disease, at this time this technology is probably best suited for patients with degenerative changes limited to a single spinal segment.49 Consequently, virtually all of the Food and Drug Administration (FDA)-sanctioned clinical trials under way are only enrolling patients for one-level disk replacements.
Compared with radiculopathy, myelopathy remains a more controversial indication for cervical arthroplasty. In general, motion preservation techniques may not represent the optimal treatment for severe or longstanding myelopathy resulting in a significantly compromised spinal cord; however, cervical disk replacement may be a reasonable surgical option for disk herniations causing more acute myelopathic signs or symptoms.
Contraindications to cervical arthroplasty include any history of tumor or infection, significant osteoporosis, or any kyphotic deformity. In addition, this surgical procedure is not recommended for patients with restricted preoperative range of motion (< 3 degrees), significant osteopenia, or any radiographic evidence of preexisting facet degeneration or adjacent segment disease.50 Because the successful implantation of any prosthesis is dependent upon the ability to obtain clear intraoperative fluoroscopic images to ensure its proper positioning, cervical arthroplasty should be avoided in patients with morbid obesity or any other condition that might preclude the unobstructed visualization of the level of interest.
 | Outcomes |
Several studies have reported the short- and intermediate-term results of cervical arthroplasty. In Bryan’s series of 48 patients involved in the original European clinical trials, 87% and 89% were considered to have had excellent, good, or fair outcomes at 1 and 2 years, respectively.47 Goffin et al evaluated 103 subjects with symptomatic cervical radiculopathy or myelopathy who underwent implantation of the Bryan cervical prosthesis (Medtronic Sofamor Danek, Memphis, TN) at a single level and another 43 who were treated at two adjacent levels.22 At 1- and 2-year follow-up, over 85% of these individuals achieved clinical success according to Odom’s criteria, and the flexion-extension range of motion per level averaged 7.9 degrees 5.3 degrees in the single-level group and 7.4 degrees 5.1 degrees in the bilevel cohort. These encouraging results have been corroborated by other clinical studies, which have also shown similar early improvements in patient-reported outcome measures using a variety of different cervical implants, including the Bryan prosthesis,50,51 the PCM (Cervitech, Rock-away, NJ),52 and the ProDisc-C.53 Although the use of cervical arthroplasty for the surgical management of myelopathy has not been universally accepted, in one series more than 90% of patients with symptomatic spinal cord compression who underwent cervical disk replacement with the Bryan prosthesis exhibited excellent or good results with an average decrease in the Nurick scale of 0.91 grades.49 Moreover, cervical arthroplasty has also been found to be associated with favorable outcomes as a treatment for adjacent segment disease as well as for patients with a history of previous cervical spine surgery who present with recurrent neural compression at the same levels.49,52,54,55
The preliminary results of cervical disk replacement have been directly compared with those following ACDF by at least two prospective, randomized clinical trials. In the series of Porchet and Metcalf patients with radiculopathy or myelopathy secondary to spondylotic changes limited to a single level of the cervical spine were randomly selected to receive one or two treatments—implantation of the Prestige II prosthesis (Medtronic Sofamor Danek) or ACDF using iliac crest autograft.56 Although significant improvement was observed in both cohorts, postoperative radiographs revealed that the disk replacements maintained motion at the operative levels without any evidence of adjacent segment degeneration. In a similar study, 33 subjects with single-level cervical disk disease were randomized to undergo either ACDF or placement of a Bryan artificial disk.57 Once again, the clinical outcome measures of both groups were equivalent with greater preservation of motion in the cervical arthroplasty patients. These preliminary findings suggest that cervical disk replacements may be a viable alternative to fusion in this patient population, but until additional prospective, randomized, controlled clinical trials with sufficient statistical power have been completed it is unlikely that total disk arthroplasty will replace ACDF as the gold standard surgical procedure for symptomatic cervical degeneration.
Cervical disk arthroplasty patients will need to be monitored for several years before any definite conclusions regarding the efficacy of this new technology may be established with any certainty. Unfortunately there is still very little data available related to the long-term performance of cervical disk replacements. A pilot clinical trial assessing the 4-year results of 14 subjects who had been implanted with the Prestige I device recently demonstrated good clinical improvement according to various patient-generated outcome questionnaires, including the Short Form-36 General Health Survey, the Neck Disability Index, and the Visual Analog Scale.58 Radiographic analysis also confirmed that motion was maintained at the operated segments during the same follow-up period. Clearly, additional longitudinal studies are necessary to evaluate the long-term function of these types of implants before they may be considered an acceptable alternative to fusion for the treatment of cervical spondylosis.
 | Authors’ Preferred Method of Surgical Management |
Even though the concept of motion preservation is intuitively appealing to physicians and patients alike, it is essential to keep in mind that as with ACDF the primary goals of cervical arthroplasty are still decompression of the neural elements, maintenance of stability, and restoration of normal sagittal alignment. We currently consider the ideal candidate for a total disk replacement to be a patient with clinical and radiographic evidence of cervical spondylosis or a herniated nucleus pulposus limited to a single level who presents with disabling radicular or myelopathic symptoms that have proven to be refractory to conservative management. In certain cases cervical arthroplasty may also be an appropriate treatment option for patients with a herniated disk resulting in the acute onset of myelopathic signs or symptoms. We do not recommend the use of these implants in individuals with multilevel degenerative disease, instability, or a history of previous cervical infection or malignancy. In addition, because the presence of a kyphotic deformity is a relative contraindication to total disk replacements,1,55,59,60 it is preferable to perform this surgical procedure in patients with normal sagittal alignment of the cervical spine.
From our own experiences with cervical arthroplasty, we acknowledge that there is a distinct learning curve associated with the proper implantation of this new technology. The head and neck of the patient should be placed in a neutral position with the shoulders taped down to facilitate intraoperative imaging. It cannot be emphasized enough that the level of interest must be able to be clearly visualized with fluoroscopy to verify the final location of the prosthesis in both the sagittal and coronal planes, so in most individuals it is difficult to insert an artificial disk caudad to the C6-7 disk space. During the exposure and decompression it is also beneficial to mark the midline by using appropriate anatomical structures such as the uncovertebral joints, pedicles, or spinous processes as another method of guiding the accurate placement of the implant. Improved operative techniques and innovations in prosthetic design will likely reduce the risk of error and minimize the amount of radiation exposure to the patient as well as the surgical staff.
Prior to implant placement, we typically perform a complete diskectomy with extensive foraminotomies bilaterally; obtaining an adequate decompression is particularly critical in the setting of advanced spondylosis because the residual motion at that segment may bring about additional degenerative changes leading to recurrent symptoms of neural compression. Although we do not necessarily remove the posterior longitudinal ligament in all cases, in patients with significant collapse of the disk space or those in whom the ligament is contracted, this structure may need to be released or even excised to achieve proper soft tissue balance and prevent a “nutcracker” effect from occurring as a result of asymmetric tensioning of the prosthesis. Finally, the operative field should be copiously irrigated after every use of the high-speed burr to facilitate the removal of bony debris and minimize the risk of developing heterotopic ossification.
 | Postoperative Care |
Although Bertagnoli reported the use of a soft cervical collar following cervical disk replacement,53 one of the advantages of motion-sparing techniques over ACDF is that external immobilization is not routinely required based on the nature of this operative intervention. Furthermore, some authors recommend a postoperative regimen of nonsteroidal anti-inflammatory medications (NSAIDs) for all cervical arthroplasty patients to reduce the risk of developing heterotopic ossification.1,49,60–63
 | Complications |
Although the incidence of intra-, peri- and postoperative morbidity following cervical disk replacement has been shown to be relatively low, this surgical procedure may result in any number of complications.14 Some of these adverse effects are also common to ACDF; for instance, cervical arthroplasty is subject to all of the adverse effects inherent to the anterior cervical approach, including vocal cord paralysis, hoarseness, dysphagia, and retropharyngeal hematoma.63,64 Other device-related complications have proven to be unique to motion-sparing technology, some of which are still evolving and have not been fully characterized or even recognized at this early juncture. Nevertheless, it may be safely assumed that many of the same challenges relevant to the field of hip and knee arthroplasty today will ultimately need to be addressed by spinal surgeons in the near future as the popularity of cervical disk replacement continues to increase.
Heterotopic ossification (HO) is an ankylosing process that may compromise the postoperative range of motion of any joint replacement, including artificial disks. In the original European Bryan disk study, 16 of 90 subjects (17.8%) were noted to have radiographic evidence of HO at 12-month follow-up, six (6.7%) of which were classified as complete grade 3 or grade 4.62 Although 10 of these patients exhibited less than 2 degrees of motion on dynamic lateral cervical x-rays, the authors did not detect a significant association between the development of HO and any clinical outcomes. In this series male gender and advanced age were identified as possible risk factors for HO after cervical arthroplasty. Several authors observed paravertebral ossifications in ~30% of cervical disk replacement patients that responded favorably to early treatment with a 2-week course of NSAIDs, with no apparent effects on prosthetic function.1,62 It has been suggested that the extensive milling of the vertebral bodies required for the placement of these implants may predispose these individuals to the formation of this ectopic bone.1,22
There is currently a paucity of data related to the issue of postoperative infection in cervical disk arthroplasty patients, but as this new technology gains wider acceptance this potential complication will inevitably need to be addressed. Like all infections, a clinical examination, imaging studies, laboratory tests, and possibly aspiration may be required to confirm this diagnosis. Although it may be reasonable to employ appropriate organism-specific antibiotics as a first-line treatment for an infected prosthesis, the majority of these cases will likely require operative intervention consisting of implant removal, thorough irrigation and debridement, and subsequent arthrodesis.65 Unfortunately the revision strategies for obtaining a solid fusion in this situation are still being developed, with no established guidelines regarding the optimal approach (anterior versus posterior), timing of surgery (immediate versus staged), and type of structural bone graft material to be used (autograft vs allograft).
The long-term survival of lower extremity total joint replacements may be compromised by the production of wear debris, which is known to activate osteolytic cytokines and initiate an inflammatory cascade that ultimately leads to aseptic loosening and prosthetic failure.1,42,43,66–69 Assuming it is retained for at least 30 years in vivo, it has been estimated that an artificial disk will undergo anywhere between 10 and 30 million cycles over the course of its lifetime.46 Biomaterial testing of several different cervical disk replacements demonstrated acceptable wear rates that are lower than those observed with hip and knee arthroplasty.66,70,71 The biological response to cervical wear particles has also been assessed using multiple animal models; although debris was noted in the periprosthetic and epidural spaces, there was no evidence of systemic inflammation, implant loosening, or neurological complications reported in any of these studies.40,66,70 Because the cervical spine is subjected to biomechanical loads that are substantially lower than those experienced by larger joints, it is conceivable that artificial disks may generate fewer debris particles and stimulate less osteolytic bone resorption than other joint replacements.25 In addition, the fibrocartilaginous cervical disk material may contain fewer macrophages and osteoclasts than synovial joints, which are likely to be more accessible to inflammatory cells.66
The importance of maintaining or restoring normal sagittal alignment following cervical spine surgery has been well documented, and any degree of kyphosis may place adjacent motion segments at greater risk for progressive degeneration.54,72–75 In the series of Johnson et al, 13 subjects treated with the Bryan prosthesis at a single level demonstrated a mean reduction in lordosis of 4.7 degrees as measured on postoperative lateral x-rays, whereas none of the patients who underwent multilevel cervical arthroplasty exhibited any kyphotic deformities.72 However, there were no significant changes in the overall sagittal alignment of the cervical spine evident in either group. The focal kyphosis that developed following single-level procedures was attributed to the milling of the end plates necessary to prepare the disk space for implant insertion. Other authors have also prospectively evaluated the postoperative sagittal curvature of cervical arthroplasty patients.33,76 Based on several different radiographic measures, the placement of a Bryan disk replacement was found to exacerbate the kyphotic orientation of that segment, most likely because of inadequate end plate preparation and asymmetric milling of the vertebral bodies. Despite the suboptimal alignment of these individuals, there was no significant correlation between kyphosis and clinical outcomes. In addition, in both of these studies the overall sagittal balance of the cervical spine was unaffected because the motion that was preserved at the treated and adjacent levels allowed for some postural compensation to occur.
 | Conclusion |
Cervical arthroplasty represents a novel surgical procedure for cervical spondylosis associated with symptoms of myelopathy, radiculopathy, or axial neck pain. Preliminary studies have suggested that the efficacy and safety profile of cervical disk replacements is at least equivalent to that of ACDF. By maintaining segmental motion, artificial disks may also decrease the incidence of adjacent-level degeneration; however, this claim has not yet been substantiated by the results of any long-term studies. The economic implications of motion sparing technology must also be assessed to evaluate its cost-effectiveness, a concern that cannot be ignored in this era of limited health care resources. Although early results have been promising, multiple prospective, randomized, controlled clinical trials with adequate follow-up will need to be performed before cervical arthroplasty may be regarded as an acceptable alternative to fusion for the treatment of cervical spondylosis.
References
1. Phillips FM, Garfin SR. Cervical disc replacement. Spine 2005;30:S27–S33
2. Lehto IJ, Tertti MO, Komu ME, Paajaen HE, Tuominen T, Korman MJ. Age related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology 1994;36:49–53
3. Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects: a prospective investigation. J Bone Joint Surg Am 1990;72:1178–1184
4. Gore DR, Sepic SB, Gardner GM. Neck pain: a long-term follow-up of 205 patients. Spine 1987;12:1–5
5. Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp 1955;96:223–224
6. Cloward RB. The anterior approach for removal of ruptured cervical discs. J Neurosurg 1958;15:602–617
7. Bose B. Anterior cervical fusion using Caspar plating: analysis of results and review of the literature. Surg Neurol 1998;49:25–31
8. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discetomy and arthrodesis for cervical radiculopathy: long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993;75:1298–1307
9. Emery SE, Bohlman HH, Bolesta MJ, Jones PK. Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy: two- to seventeen-year follow-up. J Bone Joint Surg Am 1998;80:941–950
10. Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003;28:134–139
11. Sekhon LH, Ball JR. Artificial cervical disc replacement: principles, type and techniques. Neurol India 2005;53:445–450
12. Phillips FM, Carlson G, Emery SE, Bohlman HH. Anterior cervical pseudarthrosis: natural history and treatment. Spine 1997;22:1585–1589
13. Tribus CB, Corteen DP, Zdeblick TA. The efficacy of anterior cervical plating in the management of symptomatic pseudoarthrosis of the cervical spine. Spine 1999;24:860–864
14. Pickett GE, Sekhon LH, Sears WR, Duggal N. Complications with cervical arthroplasty. J Neurosurg Spine 2006;4:98–105
15. Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand-alone cervical cages in anterior body fusion: warning. Eur Spine J 2003;12:513–516
16. Tye GW, Graham RS, Broaddus WC, Young HF. Graft subsidence after instrument-assisted cervical fusion. J Neurosurg 2002;97 (Suppl2):186–192
17. Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine 2002;27:2431–2434
18. Maiman DJ, Kumaresan S, Yoganandan N, Pintar FA. Biomechanical effect of anterior cervical spine fusion on adjacent segments. Biomed Mater Eng 1999;9:27–38
19. Wigfield CC, Skrzypiec D, Jackowski A, Adams MA. Internal stress distribution in cervical intervertebral discs: the influence of an artificial cervical joint and simulated anterior interbody fusion. J Spinal Disord Tech 2003;16:44–49
20. Matsunaga S, Kabayama S, Yamamoto T, Yone K, Sakou T, Nakanishi K. Strain on intervertebral discs after anterior cervical decompression and fusion. Spine 1999;24:670–675
21. Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs: a review of one hundred forty-six patients. Spine 1984;9:667–671
22. Goffin J, Van Calenbergh F, Van Loon J, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan Cervical Disc Prosthesis: single-level and bi-level. Spine 2003;28:2673–2678
23. Phillips FM, Carlson G, Emery SE, Bolhman HH. Anterior cervical pseudarthrosis: natural history and treatment. Spine 1997;22:1585–1589
24. Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita IC. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine 1993;18:2167–2173
25. Gore DR, Sepic SB. Anterior discectomy and fusion for painful cervical disc disease: a report of 50 patients with an average follow-up of 21 years. Spine 1998;23:2047–2051
26. Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 1999;81:519–528
27. Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion. Spine J 2004;4:190–194
28. DiAngelo DJ, Robertson JT, Metcalf NH, McVay BT, Davis RC. Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech 2003;16:314–323
29. Dmitriev AE, Cunningham BW, Nianbin H, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty. Spine 2005;30:1165–1172
30. DiAngelo DJ, Foley KT, Morrow BR, et al. In vitro biomechanics of cervical disc arthroplasty with the ProDisc-C total disc implant. Neurosurg Focus 2004;17:44–54
31. Pickett GE, Rouleau JP, Duggal N. Kinematic analysis of the cervical spine following implantation of an artificial cervical disc. Spine 2005;30:1949–1954
32. Puttlitz CM, Rousseau MA, Xu Z, Hu S, Tay BK, Lotz JC. Intervertebral disc replacement maintains cervical spine kinetics. Spine 2004;29:2809–2814
33. Pickett GE, Mtsis DK, Sekhon LH, Sears WR, Duggal N. Effects of cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus 2004;17:30–35
34. Kotani Y, Cunningham BW, Abumi K, et al. Multidirectional flexibility analysis of cervical artificial disc reconstruction: in vitro human cadaveric spine model. J Neurosurg Spine 2005;2:188–194
35. Smith HE, Wimberley DW, Vaccaro AR. Cervical arthroplasty: material properties. Neurosurg Focus 2004;17:15–21
36. Oskouian RJ, Whitehill R, Samii A, Shaffrey ME, Johnson P, Shaffrey CI. The future of spinal arthroplasty: a biomaterial perspective. Neurosurg Focus 2004;17:10–14
37. Cunningham BW, Dmitriev AE, Hu N, McAfee PC. General principles of total disc replacement arthroplasty: seventeen cases in a nonhuman primate model. Spine 2003;28:S118–S124
38. Dooris AP, Goel VK, Grosland NM, et al. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine 2001;26:E122–E129
39. Mehren C, Mayer HM. Artificial cervical disc replacement-an update. Neurol India 2005;53:440–444
40. McAfee PC, Cunningham B, Dmitriev A, et al. Cervical disc replacement-porous coated motion prosthesis: a comparative biomechanical analysis showing the key role of the posterior longitudinal ligament. Spine 2003;28:S176–S185
41. Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsanuer-Wolfe F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am 2003;85:2168–2173
42. Mabrey JD, Afsar-Keshmiri A, McClung GA II, Pember MA II, Wooldridge TM, Mauli Agrawal C. Comparison of UHMWPE particles in synovial fluid and tissues from failed THA. J Biomed Mater Res 2001;58:196–202
43. Wagner M, Wagner H. Medium-term results of modern metal-on-metal system in total hip replacement. Clin Orthop Relat Res 2000;379:123–131
44. Albert TJ, Eichenbaum MD. Goals of cervical disc replacement. Spine J 2004;4:292S-293S
45. Link HD, McAfee PC, Pimenta L. Choosing a cervical disc replacement. Spine J 2004;4:294S-302S
46. Le H, Thongtrangan I, Kim D. Historical review of cervical arthroplasty. Neurosurg Focus 2004;17:1–9
47. Bryan VE. Cervical motion segment replacement. Eur Spine J 2002;11(Suppl2):S92–S97
48. Cummins BH, Robertson JT, Gill SS. Surgical experience with an implanted artificial cervical joint. J Neurosurg 1998;88:943–948
49. Sekhon LH. Cervical arthroplasty in the management of spondylotic myelopathy. J Spinal Disord Tech 2003;16:307–313
50. Duggal N, Pickett GE, Mitsis DK, Keller JL. Early clinical and biome-chanical results following cervical arthroplasty. Neurosurg Focus 2004;17:E9
51. Sekhon L. Cervicothoracic junction arthroplasty after previous fusion surgery for adjacent segment degeneration: Case report. Neurosurgery 2005;56(1, Suppl)E205
52. Pimenta L, McAfee PC, Cappuccino A, Bellera FP, Link HD. Clinical experience with the new artificial PCM (Cervitech) disc. Spine J 2004;4:315S-321S
53. Bertagnoli R, Yue JJ, Pfeiffer F, et al. Early results after ProDisc-C cervical disc replacement. J Neurosurg Spine 2005;2:403–410
54. Wigfield C, Gill S, Nelson R, Langdon I, Metcalf N, Robertosn J. Influence of artificial cervical joint compared with fusion on adjacent-level motion it the treatment of degenerative cervical disc disease. J Neurosurg 2002;96:17–21
55. Sekhon LH, Sears W, Duggal N. Cervical arthroplasty after previous surgery: results of treating 24 discs in 15 patients. J Neurosurg Spine 2005;3:335–346
56. Porchet F, Metcalf NH. Clinical outcomes with the prestige II cervical disc: preliminary results from a prospective randomized clinical trial. Neurosurg Focus 2004;17:E6
57. Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons; a ten-year follow-up. Spine 2001;26:2463–2466
58. Robertson JT, Metcalf NH. Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results from a pilot study. Neurosurg Focus 2004;17:69–71
59. Cinotti G, Davit D, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine 1996;21:995–1000
60. Coric D, Finger F, Boltes P. Prospective randomized controlled study of the Bryan cervical disc: early clinical results from a single investigational site. J Neurosurg Spine 2006;4:31–35
61. Bell GD, Bailey SI. Anterior cervical fusion for trauma. Clin Orthop Relat Res 1977;128:155–158
62. Leung C, Casey AT, Goffin J, et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery 2005;57:759–763
63. Baron EM, Soliman AM, Gaughan JP, Simpson L, Young WF. Dysphagia, hoarseness, and unilateral true vocal fold motion impairment following anterior cervical discectomy and fusion. Ann Otol Rhinol Laryngol 2003;112:921–926
64. Bose B. Anterior cervical fusion using Caspar plating: analysis of results and review of the literature. Surg Neurol 1998;49:25–31
65. Kostuik JP. Complications and surgical revision for failed disc arthroplasty. Spine J 2004;4(6, Suppl)289S-291S
66. Anderson PA, Rouleau JP, Bryan VE. Wear analysis of the Bryan cervical disc prosthesis. Spine 2003;28:S186–S194
67. Howie DW, Haynes DR, Rogers SD, McGee MA, Pearcy MJ. The response to particulate debris. Orthop Clin North Am 1993;24:571–581
68. Jacobs JJ, Shanbhag A, Giant T. Wear debris in total joint replacement. J Am Acad Orthop Surg 1994;2:212–220
69. Goodman SB, Huie P, Song Y, et al. Cellular profile and cytokine production at prosthetic interfaces: study of tissues retrieved from revised hip and knee replacements. J Bone Joint Surg Br 1998;80:531–539
70. Anderson PA, Sasso RC, Rouleau JP, Carlson CS, Goffin J. The Bryan cervical disc: wear properties and early clinical results. Spine J 2004;4:303S-309S
71. Traynelis VC. The prestige cervical disc replacement. Spine J 2004;4:310S-314S
72. Johnson JP, Lauryssen C, Cambron HO, et al. Sagittal alignment and the Bryan cervical artificial disc. Neurosurg Focus 2004;17:1–4
73. Katsuura A, Hukuda S, Saruhashi Y, Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting degenerative process in adjacent intervertebral levels. Eur Spine J 2001;10:320–324
74. Rajshekhar V, Arunkumar MJ, Kumar SS. Changes in cervical spine curvature after uninstrumented one-and two-level corpectomy in patients with spondylotic myelopathy. Neurosurgery 2003;52:799–805
75. Wigfield CC, Gill SS, Nelson RJ, Metcalf NH, Robetson JT. The new Frenchay artificial cervical joint: results from a two-year pilot study. Spine 2002;27:2446–2452
76. Fong SY, DuPlessis SJ, Casha S, Hurlbert RJ. Design limitations of Bryan disc arthroplasty. Spine J 2006;6:233–241
< div class='tao-gold-member'>



