Chapter 21 Motor Cortex Stimulation for Relief of Chronic Pain
 MCS has been available for many years but has recently drawn interest as a viable therapy for the intractable pain patient.
MCS has been available for many years but has recently drawn interest as a viable therapy for the intractable pain patient. The Food and Drug Administration (FDA) has not approved MCS as a labeled therapy for the use of humans in the United States. Many neurosurgeons use this therapy in an off-label fashion.
The Food and Drug Administration (FDA) has not approved MCS as a labeled therapy for the use of humans in the United States. Many neurosurgeons use this therapy in an off-label fashion. Patients should be treated with spinal cord or peripheral nerve stimulation before MCS if appropriate.
Patients should be treated with spinal cord or peripheral nerve stimulation before MCS if appropriate. There is evidence supporting the use of this therapy in those patients who experience neuropathic pain but are anatomically or physiologically unable to obtain paresthetic overlap in their pain areas.
There is evidence supporting the use of this therapy in those patients who experience neuropathic pain but are anatomically or physiologically unable to obtain paresthetic overlap in their pain areas.Introduction
With the advent of modern therapies, a need to determine a proper treatment algorithm is essential to proper use of new advances. Spinal cord stimulation (SCS), peripheral nerve stimulation (PNS) and intrathecal drug delivery (IDD) are options for the patient suffering from severe pain who does not respond to new treatment options.1,2 Motor cortex stimulation (MCS) is viewed as a new therapy that may play a role in those who do not respond to the treatments noted previously. In fact, MCS is not a new option. The initial report of MCS appeared in the literature 20 years ago and that of deep brain stimulation in the early 1970s, but there is no Food and Drug Administration (FDA) approval for the use of stimulation devices over the cortex of the brain or in the brain to treat pain problems in the United States, making access to these motor cortex and deep brain stimulators for pain difficult.3,4 This chapter examines the current knowledge base regarding MCS and helps the reader understand critical issues for considering this option for patients.
Establishing Diagnosis
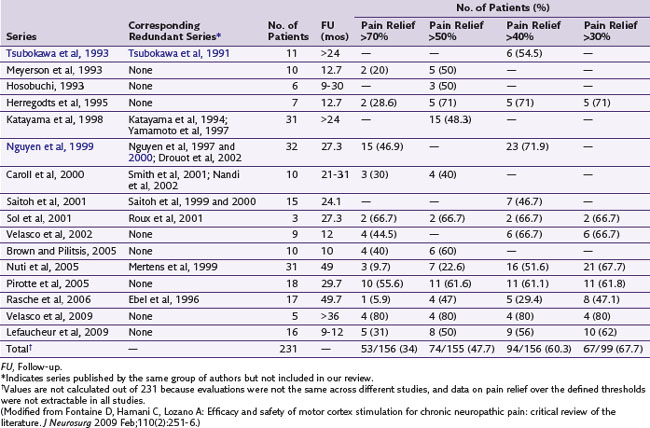
Although the original report by Tsubokawa and associates4 of MCS for chronic pain considered a group of seven patients who suffered from thalamic pain, this procedure has been used in patients presenting with varied facial pain syndromes in particular, without apparent regard to physiological presentation.5 In a review of the literature, Nguyen and associates6 did find results suggestive of modest-to-good improvement of pain in some patients with neuropathic pain. Fontaine, Hamani, and Lozano7 did an additional review and noted evidence for improvement of patients who experienced neuropathic pain on a variety of causal bases (e.g., phantom limb, central pain and plexus avulsion). Given absence of sensory paresthesias with MCS, two recent studies examined its efficacy using double-blinded stimulation parameters over a 30-day period (Velasco et al, 2009; Lefaucheur et al, 2009). Patients in both studies were then followed long term with active open-label stimulation. One study examined 16 patients with limb or facial neuropathic pain who underwent MCS; 13 were crossed over in a double-blind fashion between active and no stimulation. There were no significant differences between both groups based on visual analog scale (VAS) scores, Brief Pain Inventory (BPI) scores, McGill Pain Questionnaire–Pain Rating Index, Sickness Impact Profile (SIP), or the medication quantification scale. However, in the 12 patients who completed the open-label study, the VAS and SIP scores were significantly reduced compared to baseline. On the other hand, a 2009 study by Velasco and associates trialed five patients with refractory complex regional pain syndrome (CRPS) and implanted four. Clinical signs, VAS, and McGill Pain Scale were monitored while the implanted MCS in each patient was either turned off or on for 30 days between days 30 and 60 and days 60 and 90 following implant in a double-blind fashion. Compared to the off mode, MCS resulted in significant improvement in VAS and McGill Pain Scale and clinical improvements in allodynia, hyperalgesia and sympathetic signs. Table 21-1 examines studies using MCS for the treatment of pain.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree