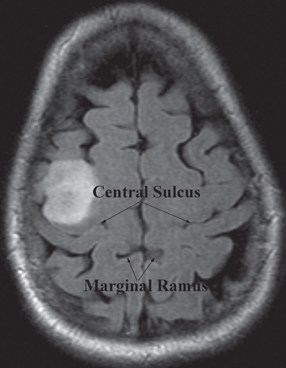
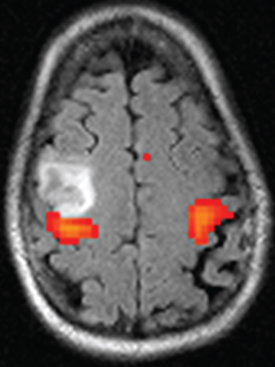
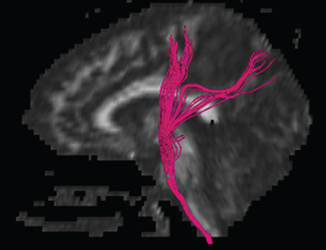
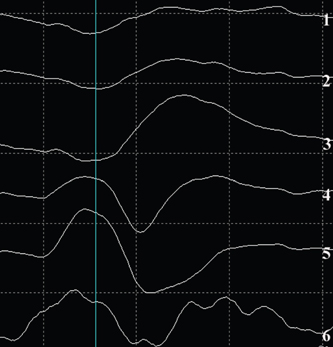
12 Motor/Sensory/Language Mapping Electrical cortical stimulation was first reported by Bartholow in 1874.1 Cushing, Penfield, Rasmussen, and others greatly refined these techniques with regard to intraoperative stimulation and recording.2–4 Additionally, refinements in these techniques have led to a better understanding of cortical organization and function.4–6 These techniques are now routinely applied to allow precise localization of structures intraoperatively.7,8 Accurate localization of functional brain regions such as rolandic cortex and language areas can potentially maximize resections of tumors, vascular lesions, and epileptic foci while reducing postoperative deficits.9 Preoperative localization with high resolution magnetic resonance imaging (MRI), functional MRI (fMRI), magnetoencephalography (MEG), and diffusion tensor imaging (DTI) allow detailed preoperative planning.10–15 These studies can also be utilized with mono-and multimodality frameless stereotactic navigation for intraoperative localization.10,12 However, it remains to be seen if preoperative imaging can completely replace intraoperative stimulation especially with regard to language function, as intraoperative mapping provides more precise localization when brain shiftlessens the accuracy of frameless navigation. This chapter is intended to provide an introduction to preoperative and intraoperative functional mapping. We will discuss the strengths and weaknesses of the various techniques as well as common pitfalls inherent in these techniques. High-resolution MRI techniques give excellent anatomical visualization and resolution. Berger et al showed that axial MRI images consistently localized the central sulcus based on the marginal ramus of the cingulated sulcus (Fig. 12.1). Parasagittal and far lateral sagittal images readily identified rolandic (sensorimotor) cortex as a functional unit.16 Quinones-Hinojosa et al showed that an anatomical analysis of the sulcal pattern of the dominant hemisphere frontal operculum correlated with intraoperative language mapping, facilitating prediction of the approximate location of Broca’s area. This information can then be used to determine the necessity of an awake craniotomy.17 Functional imaging modalities such as fMRI, MEG, and DTI can be utilized alone or in combination to localize rolandic cortex and language areas. Motor evoked responses, for example, from a finger tapping paradigm or a vibrotactile stimulator,18,19 can be utilized to localize motor cortex by fMRI (Fig. 12.2). However, fMRI localization depends on the indirect effect of neuronal activity on local blood flow. Neuronal activity changes the magnetic susceptibility of blood, producing the BOLD (blood oxygen level dependent) contrast.20 This is limited by the varying degrees of hemodynamic response to neuronal activity, especially in pathological conditions. Additionally, the BOLD activity represents blood flow from not just the activated area but also from the whole territory drained by the veins in the sampled area.20,21 Comparisons of fMRI with MEG, which depends on measurement of direct neuronal current flow, show that MEG more accurately localizes motor cortex when compared with intraoperative mapping.21–23 Recently, DTI techniques have been utilized to map white matter tracts. DTI measures the diffusion displacement properties of water in three-dimensional (3-D) space. The technique takes advantage of the difference in restricted diffusion between water perpendicular and parallel to white matter tracts. This directional variation is termed diffusion anisotrophy.15 DTI allows visualization of subcortical white matter tracts and is useful for preoperative planning in patients with intrinsic lesions (Fig. 12.3). This technique has also been utilized to identify primary motor cortex24 and has been used with other modalities such as MEG and fMRI to give a more global picture of motor cortex including subcortical tracts.12 Fig. 12.1 Axial MRI showing central sulcus in relation to the marginal ramus. Fig. 12.2 Functional MRI (fMRI) showing bilateral localization of motor cortex. Fig. 12.3 Sagittal MRI showing reconstruction of subcortical white matter tracts by diffusion tensor imaging (DTI). Amobarbitol (Amytal) Wada testing has long been the gold standard for determining language hemispheric dominance and lateralizing memory function.25–27 However, it is invasive, carrying the inherent risks of angiography. Additionally, the presence of significant vascular shunting as with a tumor or arteriovenous malformation (AVM), can make the interpretation difficult.28 Functional magnetic resonance imaging has been used extensively to localize language.29,30 It compares favorably with Wada in determining language hemispheric dominance29,30 especially in combination with MEG and DTI.31,32 However, the localization of specific language sites within the dominant hemisphere is less precise.33 This is due in part to inconsistencies in the language maps produced by different activation protocols.34 Additionally, as with all language localization, fMRI may identify language involved areas but not necessarily essential areas, which may effect surgical planning. However, recently Kamanda and colleagues reported more precise localization of specific language sites using MEG, DTI, and fMRI in combination.31,32 There have also been reports that mesial temporal memory activation detected by fMRI during complex visual scene encoding correlates with postsurgical memory outcome.13 However, the authors suggest that the reliability of these determinations needs to be further evaluated. Multimodality, preoperative imaging techniques are being combined and used in frameless stereotaxy to guide resections near eloquent cortex. Kamada et al report utilizing fMRI, MEG, in combination with DTI, and 3-D anatomical MRI to visualize both cortical and subcortical structures for use with neuronavigation-guided tumor resections. Again, the limitation of this technique is that during surgery there can be significant brain shift, negating the accuracy of these techniques. This perhaps can theoretically be addressed with intraoperative “re-registration” using intraoperative MRI. Intraoperative stimulation mapping remains the gold standard for localization of functional cortex including motor/ sensory and language areas. However, successful mapping requires a thorough understanding of the benefits and shortcomings of the various mapping modalities, as well as accurate interpretation of mapping data. As with many clinical decisions, patient selection is paramount in determining the feasibility and success of mapping. Somatosensory evoked potentials (SSEP) and cortical stimulation mapping of motor cortex can be performed under general or local anesthesia. Intraoperative language mapping and stimulation mapping of sensory cortex require an awake and cooperative patient. Awake procedures in patients with psychiatric disorders and/or developmental delay should generally be avoided. Additionally, although there is no specific age cutoff, awake mapping has been reported in children as young as 4 years of age.7,35 Physiological abnormalities such as obesity or pulmonary conditions such as sleep apnea are relative contraindications for awake procedures, as airway management is paramount. If an awake procedure is not feasible, language mapping can still be performed by placement of a subdural or epidural grid electrode for extraoperative functional mapping and seizure monitoring.36,37 This requires an additional operation, but this does not necessarily detract from the utility of this procedure. When performing SSEP monitoring under general anesthesia, halogenated anesthetic agents should not be utilized as they increase the latency of cortical SSEP.38 Agents that lead to burst suppression such as barbiturates or high-dose propofol are also generally contraindicated. Propofol in combination with a short-acting opiate such as remifentanyl have been shown to work well for these procedures.39,40 Pharmacological paralysis must be avoided or completely reversed for motor mapping. Finally, higher stimulation currents may be required for patients under general anesthesia. Awake language or sensory mapping requires an awake, cooperative patient. Generally, the patient is anesthetized for the initial scalp/bone work and then allowed to awaken for the mapping. Benzodiazopines are avoided if intraoperative electrocorticography is to be utilized. Propofol works well for this portion of the procedure as it is quickly reversible. However, it is not an adequate analgesic and so a short-acting narcotic such as remifentanyl can be added.41 Also, a generous regional field block is utilized, using short- and long-acting local anesthetics (1% lidocaine, 0.25% bupivacaine, 1:200,000 epinephrine).8 The surgeon should discuss the tolerable dose of local anesthetic for any individual patient (based on weight) with the anesthesia team. A small amount injected with a small-bore needle into the dura at the skull base near the middle meningeal artery also helps with pain control. Pin skull fixation is well tolerated with local anesthetic but is not required. When pin fixation is not used, a skull clamp can be attached to the bone edge to control the head as the patient awakens from general anesthesia, and serve as an attachment for self-retaining retractors and as a support for an electrocorticography harness. Airway issues are a prime concern with a nonsecured airway. Strategies include temporary use of a laryngeal mask or nasal airways/nasal cannula.42,43 There is also significant variability with regard to the time to awakening from propofol anesthesia after it is discontinued, as well as the patient’s cooperativeness during this awakening period. Therefore, it is often advisable to open the dura only after the patient is awake and cooperative. Urinary catheters can be placed preoperatively; however, they can be a distraction, especially to male patients. An alternative is to allow male patients to use a urine bottle on request during the procedure. Finally, with stimulation mapping there is a risk of intraoperative seizures, and possible intraoperative brain swelling. Therefore, anticonvulsant levels need to be optimized preoperatively. Intraoperative seizures can be terminated with cold irrigation solution applied to the cortex, administration of a short-acting benzodiazepine, and/or deeper levels of general anesthetic in patients with endotracheal intubation.44 In our opinion, intraoperative SSEP is an extremely useful and underutilized functional mapping technique. SSEP provide a straightforward and accurate way of identifying the primary somatosensory/postcentral gyrus, and then indirectly the motor/precentral gyrus. SSEP localization can be utilized in patients of all ages and under either general anesthesia or in an awake patient. Another primary advantage of SSEP over direct stimulation methods is that seizures cannot be evoked with SSEP.45 Intraoperative SSEP techniques involve stimulation of the median and/or tibial nerves as this will give strong cortical surface recordings.46 Stimulation is generally performed at 2 to 5 Hz, with a 0.1 to 0.3 millisecond pulse duration. The current is adjusted to give a minimal but not painful muscle twitch. The use of mechanical and thermal stimulation have also been reported.47 The stimulus provides a signal thought to be carried by the spinothalamic fibers to the thalamus by way of the medial lemniscus, subsequently ending in the contralateral somatosensory cortex. Recording from the cortical surface tends to have much higher voltages than scalp recordings. Generally, trials of 100 to 200 stimuli are needed to produce well-defined responses from somatosensory cortex. Cortical responses can be positive (P) or negative (N) when compared with the reference electrode. The number following the polarity designation is the latency of the peak in milliseconds. As an example, stimulation of the median nerve at the wrist generally produces an initial N20 component followed by a P25 peak in the contralateral cortex.48 Two types of recording configurations or montages can be utilized. A referential montage is one in which all cortical electrodes are referenced to an electrode off the cortical surface. The second type is a bipolar montage, in which each electrode is referenced to the adjacent electrodes. When using a referential montage, the somatosensory cortex is located beneath the electrode with the highest recorded amplitude. In a bipolar montage, a recorded phase reversal occurs across the somatosensory cortex (Fig. 12.4).48
Preoperative Functional Localization
Anatomical
Functional Imaging
Language Testing
Intraoperative Mapping
Frameless Navigation
Stimulation Mapping
Anesthesia
Somatosensory Evoked Potentials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








