1. Possess a practical understanding and classification of different motor events, behaviors, or phenomena that may occur in sleep.
2. Describe the polysomnographic findings of these conditions and their utility in diagnosis and differential diagnosis.
3. Define the importance of video-polysomnography and the use of other laboratory tests.
N3 slow-wave sleep (SWS); RBD occurs during REM sleep usually in the last half to third of the night; epileptic seizures are more common during nonrapid eye movement (NREM) sleep; rhythmic movement disorder usually occurs during sleep-wake transitions; and in dissociative disorders, patients may appear to be asleep, but the PSG reflects an electroencephalographic (EEG) pattern of wakefulness.
Clinical Neurophysiology Society (addition of T3 and T4 to standard PSG EEG electrodes), showed the abbreviated 8-channel montage was only slightly inferior to the 18-channel montage regarding interpreter agreement distinguishing seizure activity from nonepileptic activity (78% agreement for 8 channel and 84% agreement for 18 channel) (12). The 8-channel montage, however, was not as effective in localizing the seizure activity as the 18-channel montage (27% and 49%, respectively), especially in the temporal and parieto-occipital regions.
Table 20-1 Indications for Video-Polysomnography | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 20-2 Sample Electroencephalographic-Polysomnographic Montages | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
differentiate between organic and psychogenic etiologies (14, 15). Finally, placing a pair of electrodes along the back of the neck over the paraspinal muscles may be useful when rhythmic movement disorder is suspected (3).
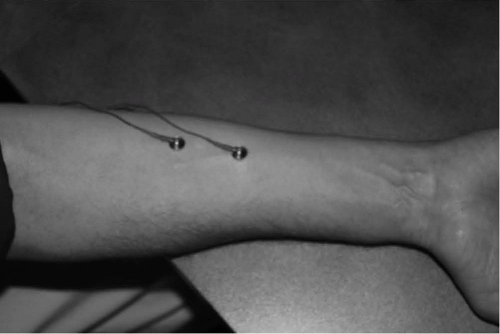 Figure 20-1 Proper placement of electrodes on the forearm flexors, 2 to 4 cm apart in line to the lateral edge of the belly of the forearm flexor (outside of the underside of the forearm). |
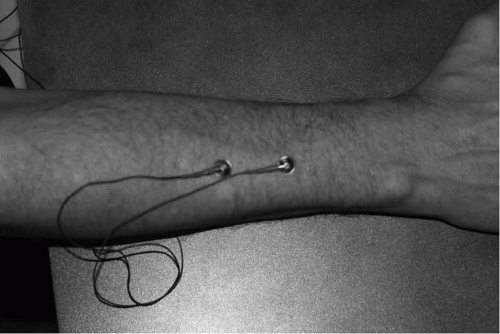 Figure 20-2 Proper placement of electrodes on the forearm extensors for evaluation of parasomnia during a sleep study. |
area. It is not always feasible for the technologist to react in time to prevent an injury to the patient. Therefore, universal seizure precautions are recommended. These precautions include keeping the bed as low to the floor as possible, removing any unnecessary equipment from the room, and maintaining constant observation. Some sleep centers recommend the use of bed rails or pillows to restrict the mobility of the patient during a nocturnal spell. Although these measures can impede the patient from leaving the bed, they can also trip the patient or (in case of bed rails) even increase the height from which the patient jumps out of bed. Thus, in some instances, these safety precautions may actually increase the likelihood of a patient injury (26). Individual sleep centers will need to develop their own policy regarding the use of rails or pillows. Patient injuries from striking sharp
corners of furniture can be minimized by padding them with foam corners commercially available for nurseries. Any breakable room decorations (lamps, vases, etc.) that may injure the patient should be removed from the room. In extreme cases such as with a history of the patient diving out of bed, padding or a second mattress placed on the floor next to the patient’s bed may further reduce the risk of injury.
Table 20-3 Movement Disorders Stage and Characteristics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





