A. Failure of motor control at NREM sleep onset
1. Physiological
a. Physiological body movements and postural shifts
b. Physiological hypnic myoclonus
c. Hypnic jerks
d. Hypnagogic foot tremor
e. Rhythmic limb movements
2. Pathological
a. Intensified hypnic jerks
b. Rhythmic movement disorder
c. Propriospinal myoclonus at sleep onset
B. Failure of motor control during NREM sleep
1. Partial arousal disorders
a. Confusional arousals
b. Sleep walking
c. Sleep terror
2. Others
a. Alternating leg muscle activity
b. Periodic limb movements in sleep
C. Failure of motor control during REM sleep
1. Physiological
a. Phasic muscle bursts includingfragmentary hypnic myoclonus
b. Phasic tongue movements
c. Sleep paralysis
2. Pathological
a. RBD
b. Sleep paralysis with narcolepsy
c. Familial sleep paralysis
d. Cataplexy
D. Failure of motor control in both NREM and REM sleep
a. Rhythmic movement disorder
b. Catathrenia
c. Excessive fragmentary myoclonus
d. Sleep bruxism
e. Upper airway obstructive sleep apnea syndrome
E. Failure of motor controlat sleep offset
a. Sleep paralysis
b. Hypnopompic hallucination
c. Sleep inertia (“sleep drunkenness”)
F. Diurnal movement disorders persisting in sleep
1. Usually persisting during sleep
a. Symptomatic palatal tremor
2. Frequently persisting during sleep
a. Spinal and propriospinal myoclonus
b. Tics in Tourette’s syndrome
c. Hemifacial spasm
d. Hyperekplexia
3. Sometimes persisting during sleep
a. Tremor
b. Chorea
c. Dystonia
d. Hemiballismus
Abnormal movements that may occur during sleep include motor parasomnias (nonrapid eye movement [non-REM], rapid eye movement [REM], and other parasomnias), sleep-related movement disorders (a separate category was included in the ICSD-2) [4], isolated sleep-related motor symptoms (apparently normal variants), miscellaneous nocturnal motor activities, and traditional diurnal involuntary movements persisting during sleep. Many of these nocturnal motor events may be mistaken for nocturnal seizures (traditionally not classified with movement disorders), especially myoclonic seizures and nocturnal frontal lobe epilepsy (NFLE) or what was originally termed nocturnal paroxysmal dystonia (NPD). Figure 29.1 schematically shows the most common sleep-related movements which need to be considered and differentiated from each other. In this chapter, we describe the evolution and historical milestones of some of those sleep-related movements including the ICSD-3 [4] category of sleep-related movement disorders as well as some diurnal movements persisting during sleep. For a historical account of RLS/WillisEkbom disease , non-REM parasomnias, REM behavior disorder (RBD), and nightmare disorders (REM parasomnias), see other chapters of this book.
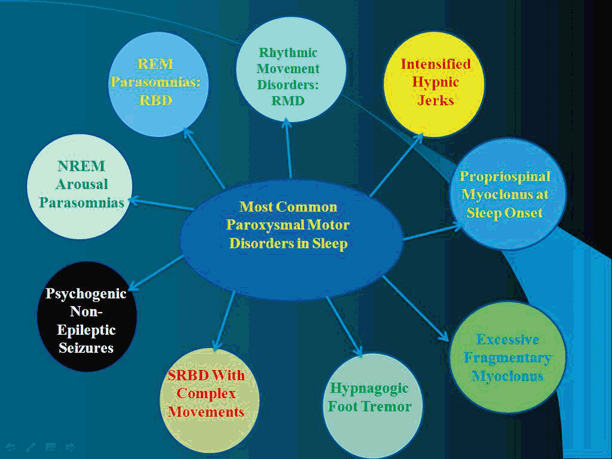

Fig. 29.1
Most common paroxysmal motor disorders in sleep
Szymanski [5] first attempted to study body motility during sleep using rudimentary actigraphs (“sensitive bed” principle of movement registration) in 1914. It was revealed for the first time that sleep is not just a period of rest and repose but there are interruptions due to body movements. Later, polysomnography (PSG) and particularly video-PSG studies clearly documented physiological body movements and postural shifts during sleep. Gastaut and collaborators from France [6, 7] were the first to study sleep-related normal and abnormal movements using polygraphic technique with multiple surface electromyography (EMG) recordings. Shortly thereafter, Lugaresi and coinvestigators from Italy [8, 9] made important polysomnographic contributions on the evolution of abnormal movements during sleep. Arousals (periods of interruptions from sleep to brief awakenings lasting up to 14 s or less) are often associated with body movements, and these may precede or follow postural shifts. Arousals may be both physiological (e.g., associated with body shifts in normal individuals) and pathological (e.g., on termination of sleep apneic–hypopnic episodes or associated with periodic limb movements in sleep, PLMS) . Body movements and postural shifts are frequent at sleep onset and fairly common in stage N1, occur less frequently in stage N2, and are rarely seen in stage N3 but again may be frequent in stage REM [10]. Movements vary not only with sleep stages but also with age. In one study [11], postural shifts during sleep decreased from 4.7/h in 8–12–year-olds to 2.1/h in the elderly (65–80 years). Body motility was among the earliest physiological characteristics of sleep studied [12]. All night polygraphic recordings showed a significant temporal relationship between preceding K-complexes and body movements in the early physiological studies of Gastaut and Broughton [13], and Sassin and Johnson [14].
Disorders of Failure of Motor Control at Non-REM Sleep Onset
Physiological
Physiological Hypnic Myoclonus
The term physiological hypnic myoclonus (PHM) was first coined by De Lisi in 1932 to describe brief asynchronous, asymmetric, and aperiodic muscle twitches during sleep in all body muscles of humans and domestic animals resembling fasciculations seen prominently in face and distal body parts (e.g., face, lips, fingers, and toes) [15]. PHM is also known as physiological fragmentary hypnic myoclonus and is seen prominently in babies and infants . Quantitative study by Dagnino et al. in 1969 [16] and Montagna and collaborators [17] in 1988 showed the maximum occurrence of these twitches in stage N1 and REM sleep, decreasing progressively in stages N2 and N3. Presence of PHM also during relaxed wakefulness challenges the term hypnic myoclonus [17, 18]; however, it should be noted that propriospinal myoclonus (PSM) at sleep onset and intensified hypnic jerks in many patients [19] are present in relaxed wakefulness before sleep onset. The origin of PHM remains controversial. Facilitatory reticulospinal tract [20], pontine tegmentum [21], and corticospinal tract [16] have all been suggested as the generator of PHM. These movements are physiological without disrupting sleep architecture and these require no treatment.
Hypnic Jerks Including Intensified Hypnic Jerks
Hypnic jerks are sudden, brief contractions of the body that occur at sleep onset and are due to excitation of motor centers. They are physiological and occur in up to 70 % of the population at some point in their adult lives. They are often accompanied by a sensation of falling. The earliest mention of this phenomenon is credited to Weir Mitchell [22] (Fig. 29.2), who in 1890 described insomnia occurring as a result of hypnic jerks . Oswald [23] first described the electroencephalography (EEG) correlates of hypnic jerks. In 1965, Gastaut and Broughton performed the first polygraphic study of hypnic jerks [6, 13]. It was not until 1988 that Broughton [24] coined the term “intensified hypnic jerks” to describe the clinical phenomenon of sleep-onset insomnia caused by accentuated and disruptive hypnic jerks occurring at sleep onset. More recently, Chokroverty et al. [19] performed a polysomnographic and polymyographic analysis of ten patients with intensified hypnic jerks and identified four patterns of propagation: synchronous and symmetrical patterned muscle bursts between the two sides and agonist–antagonist muscles similar to those noted in audiogenic startle reflex, reticular reflex myoclonus, dystonic myoclonus, and pyramidal myoclonus with rostrocaudal propagation of muscle bursts.
Fig. 29.2
Silas Weird Mitchell (1829–1914)
Hypnagogic Foot Tremor
HFT is defined as rhythmic contractions of foot and leg occurring during sleep onset generally bilaterally but asynchronously at a frequency of 0.3–4 Hz, and was first described by Broughton in 1986 [24]. Wichniak and colleagues [25] later performed PSG on 375 consecutive subjects and found HFT (which they called “rhythmic feet movements while falling asleep” and described as rhythmic, oscillating movements of the whole foot or toes) in 7.5 %. The clinical significance of HFT remains undetermined requiring no treatment.
Rhythmic Limb Movements in Sleep and Wakefulness
Rhythmic leg movements in non-REM (NREM) sleep, REM sleep, and wakefulness are frequently noted during PSG recordings in the sleep laboratory [26]. Yang and Winkelman [27] recently reported “high-frequency leg movements” in a retrospective study to describe similar phenomena seen in both wakefulness (two-thirds) and sleep (one-third). The significance of these leg movements remains undetermined. There have been brief recent reports of limb and body movements, both rhythmic and complex, on termination of apneas/hypopneas, eliminated by positive pressure therapy [28, 29, 30].
Pathological
Rhythmic Movement Disorder
RMD is characterized by repetitive, often dramatic and stereotyped, rhythmic movements involving large muscle groups, occurring predominantly during sleep onset or during sleep–wake transitions, at a frequency of 0.5–2 Hz [4, 31]. In 1905, Zappert [32] described nocturnal rhythmic head banging in six children and coined the term jactatio capitis nocturna. Between 1905 and 1928, Cruchet [33, 34], who used the term rhythmie du sommeil, published several observations in French, among which was acknowledgment that credit for the earliest description of this phenomenon should most likely go to Wepfer, who reported a case of rhythmic head movement activity that occurred at night as far back as 1727. There was a report, as early as 1880, by Mary Putnam-Jacobi [35], of a case of nocturnal rotary movement in an 18-month-old boy which appears to be the first clear description of what can be considered to be a case of RMD published in a popular journal of the nineteenth century. RMD generally presents before 18 months of age with head banging, head and body rolling, and body rocking occurring immediately before sleep during relaxed wakefulness continuing into stage N1 and sometimes into stage N2. Leg rolling and leg banging have also been described. RMD is generally benign and the child usually outgrows the movements by the second or third year of life but sometimes may persist into adolescence and adulthood when treatment may be needed. The first line of treatment should be behavioral therapy and in severe cases with potential for inflicting injury clonazepam (0.5–1 mg nightly) or imipramine (10 mg at night) may be helpful [36]. Protective measures should be used in cases with violent movements .
PSM at Sleep Onset
PSM , representing myoclonic activity arising in the relaxation period preceding sleep onset, was first described in three patients in 1997 by Montagna et al. [37] They performed polygraphic studies that showed that the myoclonic activity began in spinally innervated muscles, propagating at low speed to rostral and caudal muscular segments, and hypothesized that a spinal generator may be facilitated by changes in supraspinal control related to vigilance levels. They identified it as a potential cause of severe anxiety and insomnia. Subsequently, the same group [38] described another five patients of PSM at wake–sleep transition. Most cases are idiopathic without any structural lesion. PSM has also been described more recently by the same group in three patients with RLS (recently renamed Willi–-Ekbom disease) [39]. Manconi et al. [40] described a severe and uncommon case of PSM during wake–sleep transition following a vertebral fracture of T11. The uncommon features of this case include focal myoclonic activity in the axial muscles during stable sleep and later progression into a myoclonic status indicating a very high spinal cord excitability. Recently, a case of PSM at sleep onset was described in an Asian woman from Singapore [41]. The pathophysiological mechanism of PSM at wake–sleep transition stage (predormitum as suggested by Critchley [42]) is hypothesized to be due to the lack of supraspinal inhibitory control at this stage, with resultant spinal cord hyperexcitability propagated through propriospinal pathways [37, 38]. The treatment of this condition is challenging and some cases respond to clonazepam , zonisamide, and other antiepileptic drugs used in the classic PSM [36] .
Failure of Motor Control During NREM Sleep
Alternating Limb Muscle Activity During Sleep
Alternating leg muscle activation (ALMA), first described by Chervin and colleagues in 2003, is characterized by brief activation of the anterior tibialis muscle in one leg alternating with similar activation in the other leg, usually lasting up to 20 s and occurring in all sleep stages, but particularly during arousals [43]. In 2006, Consentino and colleagues [44] described a patient with ALMA whose condition responded to pramipexole. Our group [45] documented ALMA in wakefulness, all stages of NREM, and also though less in REM sleep, in patients with a variety of sleep disorders. We observed ALMA also in gastrocnemius and sometimes in quadriceps muscles alternating between two sides. The significance of ALMA remains undetermined but may be a variant of PLMS.
Periodic Limb Movements in Sleep
PLMS is a well-known polysomnographic finding, characterized by repetitive, often stereotyped, and sometimes complex involuntary movements of the limbs, trunk, and occasionally cranially innervated muscles . The first description of this condition was in 1953 by Symonds [46], who used the term “nocturnal myoclonus” to distinguish the phenomenon from hypnic jerking, which he described as “nocturnal jerking.” It had been his opinion that both these conditions were associated with an increased risk of epilepsy . A review of his clinical description suggests that Symonds included cases of familial RLS, sleep starts, and myoclonic epilepsy. It was not until 1980 that the term “nocturnal myoclonus” was replaced by “periodic limb movements in sleep” after Coleman et al. [47] clarified that the movements were too prolonged to be classified as myoclonic, and that there was no epileptiform potential associated with them. The association between impaired renal function and PLMS was established in 1985 [48]. The first polygraphic study of PLMS was published by Lugaresi and Coccagna and their collaborators [9, 49, 50] and they demonstrated the common association with RLS as well as the presence of PLMS in normal subjects. An electrophysiological study of PLMS was published later by Wechsler et al. in 1986 [51]. Studying lower limb H-waves, blink responses, and median nerve somatosensory-evoked responses, they postulated that PLMS was likely secondary to a disorder of the central nervous system producing increased excitability of segmental reflexes at the pontine level or rostral to it. In 2001, Provini et al. [52] studied the motor pattern of PLMS neurophysiologically with EMG/nerve conduction studies, somatosensory-evoked potentials, and transcranial magnetic stimulation, all of which were normal . They found that in PLMS, leg muscles were most frequently involved, often with alternation of sides. Axial muscles were rarely involved and upper limb muscles were involved only sometimes. The tibialis anterior muscle was the most frequent to show the onset of PLMS. There was no constant recruitment pattern from one PLMS episode to another, even in the same patient. There was no orderly caudal or rostral spread of the EMG activity. They speculated about the presence of several generators at various levels of the spinal cord, released by a supraspinal generator . In 2004, de Weerd and colleagues [53] studied activity patterns in patients with PLMS and found that the classic pattern of movement (extensor digitorum brevis, EDB–tibialis anterior, TA–biceps femoris, BF–tensor fascia lata, TFL) or its direct variants was found in only 12 % of the total 469 movements analyzed. The most frequent sequences were characterized by contraction of only the TA, TA–EDB only, or TA–EDB followed by all other combinations (32 %). In 1991, Ali et al. [54] reported the first observation of sympathetic hyperactivity caused by PLMS, noting a mean increase in systolic blood pressure following leg movements of 23 %, comparable to that noted in obstructive sleep apnea . In 1993, Pollmächer and Schulz [55] reviewed PSG characteristics of PLMS and found them most frequent at sleep–wake transition, attenuated during deep NREM sleep and even more during REM sleep. The first description of periodic arm movements in association with periodic leg movements in sleep was made by Chabli et al. in 2000, [56] when they studied 15 cases of patients with RLS who exhibited this phenomenon. That same year, Nofzinger and colleagues [57] described the distinctive characteristic of bupropion of improving rather than worsening PLMS unlike other antidepressants. It is notable that bupropion has some dopaminergic function which may be responsible for this effect. There is currently no scientific evidence that PLMS per se are responsible for insomnia or hypersomnia but they are noted in at least 80 % of cases of RLS. The scoring criteria for PLMS have been updated recently [31] .
Failure of Motor Control During REM Sleep
Phasic Muscle Movements in REM Sleep (Including Rhythmic Tongue Movements)
REM sleep is associated with a variety of phasic phenomena, such as phasic eye movements, body and limb movements, transient muscle bursts (fragmentary myoclonus), and irregular heart rate and respiration. Less-well-defined and less commonly recognized phasic events in REM sleep include spontaneous middle ear muscle activity (MEMA) in human described by Pessah and Roffwarg in 1972 [58], and phasic movements of the tongue. In 1980, Megirian et al. [59] described rhythmic activity of the tongue in rats during REM sleep. Similar complex tongue movements during REM sleep in human occurring irregularly and lasting for 2–10 s were reported by Chokroverty in 1980 [60]. These complex movements may counteract posterior displacement of the tongue, which may otherwise occur in supine REM sleep because of genioglossal hypotonia, thus functioning as nature’s defense against upper airway obstructive sleep apnea during REM sleep.
Failure of Motor Control in both NREM and REM Sleep
Catathrenia
Catathrenia , or nocturnal groaning, is a relatively new isolated symptom characterized by loud expiratory vocalization, whose exact pitch and timber may vary from individual to individual but is fairly stereotyped in a given patient. While far more frequent in REM sleep, it may also occur in NREM sleep and alternate with normal breathing. It was actually first described by Pevernagie et al. [61], but was first named by Vetrugno et al. [62] in 2001. The same group subsequently reported in 2007 [63] that the groaning was accompanied by disproportionately prolonged expiration causing reduced tidal volume and bradypnea without oxygen desaturation, and that patients experienced no additional symptoms after a mean follow-up of 4.9 years. They speculated that catathrenia was due to persistence of a vestigial type of breathing pattern. In 2011, Ott and colleagues [64] performed laryngoscopy under deep sedation in a patient with catathrenia and found that while the glottis was open at inspiration, there was subtotal closure of the glottis at expiration, resulting in the characteristic groaning. The following year, Koo et al. [65] performed acoustic analysis of catathrenia and found that it had morphologic regularity, with two types of sound pitches (either a monotonous sinusoidal pattern or a sawtooth-shaped signal with higher fundamental frequency), as opposed to snoring which was distinct from catathrenia and had an irregular signal. Several authors have reported the efficacy of continuous positive airway pressure (CPAP) in treating this benign but socially awkward condition [66, 67] .
Excessive Fragmentary Myoclonus
Excessive fragmentary myoclonus (EFM) is a predominantly PSG finding, currently described as being present if EMG bursts of at least 150 ms occur at a rate of at least 5/min sustained over 20 min of NREM sleep [31]. The first description of EFM was published by Broughton and colleagues in 1985, based on the PSG findings in NREM sleep in 38 consecutive patients [68]. They reported an association with sleep-related respiratory problems, PLMS, narcolepsy, insomnia, and excessive daytime sleepiness. Prior to this, Broughton and Tolentino [69] described what they called fragmentary pathologic myoclonus in a 42-year-old man presenting with excessive daytime sleepiness. In 1993, Lins et al. [70] reported that EFM occurred at high rates in all stages of sleep (including REM) but at a somewhat lower frequency in slow-wave sleep (SWS) explaining, as well, a significantly lower rate in the first hour after onset compared to later hours. More recently, Hoque et al. [71] reported that EFM rates increase with SWS and total REM with the highest EFM rates occurring during phasic REM. The clinical significance and pathophysiology of EFM remain undetermined. A neurophysiologic analysis by Vetrugno et al. failed to disclose any cortical prepotential on EEG–EMG backaveraging suggesting a subcortical origin [72].
Sleep Bruxism
While nocturnal bruxism may occur in patients with daytime tooth grinding, it is clearly a distinct entity in its own right, and can lead to excessive dental wear, autonomic arousals, and sleep fragmentation. One of the earliest works regarding the phenomenon was in 1964 by Reding [73], who predicted a relationship between sleep bruxism and dreaming based on its occurrence in REM sleep. The close association between bruxism and REM sleep was further commented upon by Clarke and Townsend in 1984 [74]. Shortly thereafter, Wieselmann and colleagues [75] analyzed the duration and amount of pressing and grinding jaw movements in ten patients with bruxism, and found that the highest level of activity was during stage N3 and wakefulness, with no difference seen with regard to percentages of the sleep stages. In 2001, Lavigne and colleagues [76] coined the term “rhythmic masticatory muscle activity” (RMMA) in sleep, and found that while the number of episodes of RMMA was comparable between bruxers and controls, the number of EMG bursts per episode was more frequent in the former group. In 2008, Manconi et al. [77] published an interesting case report of a patient with sleep bruxism and catathrenia occurring in a synchronized fashion. They hypothesized about the presence of a common trigger mechanism for both phenomena.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





