Chapter 11 Multicultural aspects of hearing loss
A hearing impairment may be caused by genetic factors or nongenetic factors, such as infections and ototoxic drugs. In a young child, hearing loss can impede normal development of language and speech and, subsequently, affect education. In the adult, it may result in vocational and economic difficulties and lead to social isolation and stigmatization. The increased life expectancy and the growing world population suggest that, in the future, there will be greater numbers of persons with hearing impairment unless decisive public health action is taken to reduce and eventually eliminate preventable hearing impairment and disability by implementing appropriate preventive measures (World Health Organization [WHO], 2001).
Although deafness can occur in persons of all races, genders, and ages, there are differences among ethnic groups depending on the etiological factor under review. For instance, the leading cause of deafness in the Hispanic community is maternal rubella, but in the African American community, it is meningitis (Schildroth & Hotto, 1995). To some extent, health differences and this disparity appear to be related to socioeconomic status. On average, white Americans have better access to the social and economic resources necessary for a healthy environment and lifestyle and better access to preventive medical services than minorities.
The WHO’s Global Burden of Disease (GBD) provides a comprehensive and comparable avenue to assess the impact of an illness or injury. The computation takes into account the incidence, average duration of an illness, and the relative risk of mortality. The causes of the global burden of disease are assessed according to the percentage of total disability adjusted life years (DALYs) in the world attributable to each etiological factor. Thus DALYs are a measure of the years of healthy life lost (YLL) and the years lived with disability (YLD). The data used to estimate YLD are incidence of disability, duration of disability, and a weight factor that reflects the severity of the disease. Since 2001, the WHO has included adult-onset hearing loss in the tables of the global burden of disease in the World Health Report. Adult-onset hearing impairment ranks third among contributors to YLD. YLL for hearing loss is zero; therefore, the DALY burden comes from YLD. GBD was first put into use in 1990, and last revised in 2004. DALY estimates will be revised again after the release of the Global Burden of Disease 2010 study (WHO, 2000; Smith, 2008).
It has been estimated that the lifetime costs of severe to profound hearing loss in the United States are about $297,000 per year; 21% of these costs are incurred in the provision of special education services (Mohr et al., 2000). It has been further estimated that with reduced work productivity, hearing health care, and special education, lifetime costs for those with severe to profound hearing loss acquired prelingually are about $1,000,000 (McPherson, 2008).
Incidence of hearing impairment in the world
Epidemiology of hearing loss and deafness in the united states
The number of adult Americans with hearing loss has increased drastically, possibly because of an aging population and increased exposure to noise. Agrawal and colleagues (2008) administered a national cross-sectional survey to 5742 adults aged 20 to 69 years, who participated in the audiometric component of the National Health and Nutrition Examination Survey. They reported that in 2003-2004, 16.1% of American adults, or approximately 29 million, had hearing loss in the speech frequencies, whereas in the youngest age group that they tested (20 to 29 years), 8.5% demonstrated hearing loss. Further, they observed that the odds of having a hearing loss were 5.5 times greater in men than in women and 70% greater in whites than in blacks participating in the survey. Also, according to the survey, smoking, noise exposure, and cardiovascular risk increased the prevalence of hearing loss.
Age, gender, and race
The U.S. Department of Health and Human Services (2010) reported the results of the National Health Interview Survey, 2009. Overall, 15% of adults, who were 18 years and older, reported having difficulty with hearing (without a hearing aid), and men were more likely to have this problem than women. Age was positively correlated with hearing difficulties; that is, as age increased, so did the percentage of adults with this problem. There was a higher incidence of trouble in hearing in non-Hispanic white adults (16%) than in non-Hispanic black (10%) and Hispanic (10%) adults. Persons who were younger than 65 years and those covered by Medicaid were more likely to have hearing problems.
Mitchell (2006) provided an independent analysis of the federal government’s Survey of Income and Program Participation (SIPP) data files for 2001 and reported that across all age groups, approximately 1,000,000 people (0.38% of the population) were “functionally deaf,”and 10,000,000 persons were hard of hearing. Of these, more than half were 65 years or older, and less than 4% were younger than 18 years. However, these findings included only those who reported having difficulty in hearing normal conversation with or without the use of a hearing aid, and excluded those persons with hearing loss whose hearing was affected “outside the range and circumstances of normal conversation.”
Similarly, Schoenborn and Heyman (2009) used data from the 2004-2007 National Health Interview Survey and reported on selected health characteristics of four groups of older adults (55 to 85 years or older). They found that health disparities existed across subgroups of older adults and that these appeared to vary with age. For instance, the prevalence of hearing impairment increased with age. Approximately 31.6% of persons 55 years and older, 23% of persons aged 55 to 64 years, and 62.1% of those 85 years and older demonstrated some degree of hearing impairment. Schoenborn and Heyman also reported gender differences; in the 55- to 64-year age group, men (32.2%) were twice as likely as women (16.4%) to have hearing impairment, whereas in the 75- to 84-year age group, men (53.5%) were 1.5 times more likely than women (37.0%) to have hearing impairment. Finally, in the 85 years and older group, there was a smaller difference between the prevalence of hearing loss in men (69.0%) and women (58.5%).
Based on data from the Family Core and the Sample Core components of the 2004-2006 National Interview Surveys conducted by the CDC’s National Center for Health Statistics, it was estimated that non-Hispanics, American Indians, and Alaska Natives were two to four times more likely to have a lot of trouble hearing or to be deaf than white, Hispanic, black, or Asian adults (Barnes et al., 2008). Schoenborn and Heyman (2009) also reported racial differences; among those 65 years and older, 4 out of 10 white adults (41.6%) had hearing impairment, compared with 2 out of 10 non-Hispanic black adults (23.6%), and three out of 10 non-Hispanic Asian (30.1%) and Hispanic (28.2%) adults.
Pratt and associates (2009) also examined the impact of age and race on the prevalence and severity of hearing loss in elder adults (72 to 96 years). They concluded that hearing loss was greater in the eighth than seventh decade of life, and that race and gender influenced the decline. These findings are consistent with those of Helzner and colleagues (2005) and Dillon and associates (2010). Helzner and colleagues reported a prevalence of hearing loss of 59.9% and prevalence of high-frequency hearing loss of 76.9% in 2052 adults aged 73 to 84 years. The incidence was higher in white males and females than in black males and females. Older age, race, diabetes mellitus, cerebrovascular disease, smoking, poor cognitive status, exposure to occupational noise, and ear surgery were associated with hearing loss. Race- and gender-specific risk factors included hypertension and occupational noise exposure in white men, poor cognitive factors and smoking in black women, low total hip bone mineral density in black men. Similarly, Dillon and associates reported (1) sensory impairment increased with age; (2) one out of four persons older than 70 years reported hearing loss; (3) older men were more likely to have hearing impairment than older women; (4) non-Hispanic white and Mexican American persons were more likely to have hearing loss than non-Hispanic black persons, and (5) approximately 70% of older Americans with hearing loss in at least one ear potentially could benefit from using a hearing aid but did not use amplification.
Hearing loss and deafness in children and youth
Hearing loss in the pediatric population has a deleterious effect on communication skills, social skills, and educational achievement. Shargorodsky and coworkers (2010) examined the current prevalence of hearing loss in U.S. adolescents and examined the change in incidence over time. They examined data from the Third National Health and Nutrition Examination Survey (1988-1994) and from the 2005-2006 survey. They reported that the prevalence of hearing loss in 12- to 19-year-olds had increased significantly from 14.9% in 1988-1994 to 19.5% in 2005-2006. Unilateral hearing loss was more prevalent in 2005-2006 (14%) than in 1988-1994 (11.1%). Further, the high frequencies were more involved in 2005-2006 (16.4%) than in 1988-1994 (12.8%). Also, it was reported that children from families below the federal poverty threshold (23.6%) were more likely to have hearing loss than children from above the poverty threshold (18.4%).
Based on questions from the Family Core and Sample Child Core questionnaires of the 2001-2007 National Health Interview Survey (NHIS), Pastor and coworkers (2009) reported estimates of functional difficulties related to “sensory difficulties” (vision and hearing) in school-aged children (5 to 17 years). They reported that 3% of children had sensory difficulty, of whom 11% had difficulty only in hearing, 88% only in seeing, and 1% in both hearing and seeing. Although a child’s age, gender, race/ethnicity had negligible or insignificant impact on the prevalence of sensory deficits, certain other trends were observed. For instance, poor children (5%) were more likely to have sensory difficulty than children who were from middle- and upper-income families. Children who had public health insurance (4%) or who were uninsured (4%) were more likely to have sensory deficits than children with private insurance (3%). Also children in “mother-only” families (4%) were more likely to have sensory deficits than children from two-parent (3%) families.
In its 29th Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act (IDEA), 2007, the U.S. Department of Education (2010) reported that in 2005, 0.1% of students who were 3 to 5 years or 18 to 21 years of age, and 0.5% of students who were 6 to 17 years of age, who had hearing impairment, were served under IDEA, Part B. Risk ratios compare the proportion of a particular racial/ethnic group served under Part B to the proportion served among other racial/ethnic groups combined. In 2005, the risk ratios for children 6 to 21 years of age served under IDEA, Part B, for hearing impairment, differentiated by ethnicity, were as follows: American Indian/Alaska Native (1.34), Hispanic (1.28), Asian/Pacific Islander (1.20), black not Hispanic (1.10), and white not Hispanic (0.77). That is, American Indian/Alaska Native students were 1.34 times more likely to be served under IDEA, Part B, for hearing impairment. Further, it was reported that in 2005, only 13.5% of students were educated in separate environments (e.g., Schools for the Deaf), whereas 48.3% students with hearing impairments spent most of the school day in regular classrooms (<21% of the day outside the regular classroom). The percentage of students with hearing impairment graduating with a regular high school diploma showed some improvement between 1995-1996 (58.8%) and 2004-2005 (69.6%). Students with hearing impairment had one of the lowest dropout rates of all disabilities. Twenty percent of students with hearing impairment participated in their state accountability testing without accommodations or modifications, 63% participated with accommodations or modifications, 16% participated in alternate assessments, and only 1% did not participate in standardized tests or alternate assessments. The mean standard scores for assessments in 2001-2002 and 2003-2004 showed that secondary school students with hearing impairment exhibited stronger mathematics calculation skills (mean standard score = 91.5) than knowledge/skills in applied problems (83.9), social studies (80.5), science (75.4), passage comprehension (75.6), and synonyms/antonyms (84.1).
Data from the GRI Annual Survey between 2003 and 2008 demonstrated that the gender ratio of males to females has remained fairly stable; on an average, males make up 54.2% of this population and females 45.8%. Data on the geographic region where the students were located, consistently (for each of the 5 years) showed the highest number of hearing-impaired students (average of 5 years = 14,048.8) residing in the South, whereas the lowest number (average of 5 years = 5672.6) resided in the Northeast. Table 11-1 provides the national counts of deaf and hard-of-hearing children by age. The total number of children younger than 3 years show definite increases each year from 2003-2004 through 2006-2007. On the other hand, fewer students 18 years and older appear to have enrolled after 2003-2004; there was a significant reduction in the number of students in 2004-2005, and the lower count appears to have been maintained in subsequent years.
TABLE 11-1 Number of Children and Youth by Age Who Were Deaf or Hard of Hearing during the 2003 to 2008 School Years
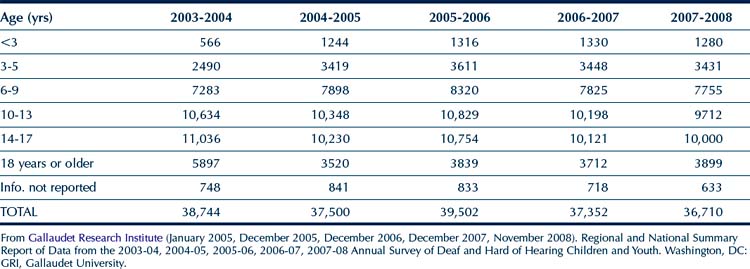
Table 11-2 summarizes the instructional setting of the students for the years 2003-2004, 2004-2005, 2005-2006, 2006-2007, and 2007-2008. For each of these years, consistent with IDEA, the highest percentage of students were reported to be studying in a regular school setting with hearing students, and this number shows a gradual increment for each of the years. On the other hand, there is a steady decrease in the number of students being taught in self-contained classrooms.
TABLE 11-2 Percentage of Children and Youth with Hearing Impairment in Types of Instructional Settings during the 2003 to 2008 School Years*
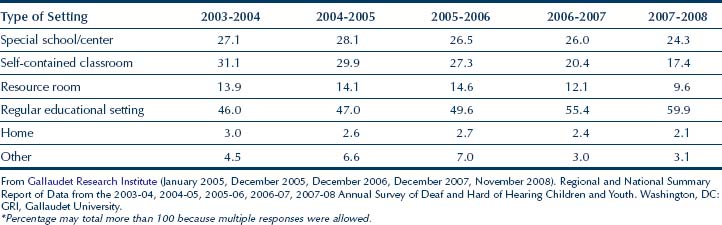
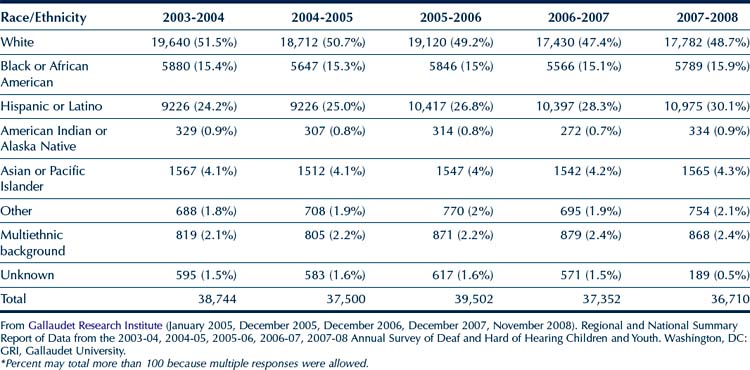
The racial/ethnic breakdown of the population in the GRI Survey is provided in Table 11-3. Except for a small increase in the Hispanic/Latino students and a slight decrease in the number of white students, the percentage representation of the other racial/ethnic groups in this population has remained relatively stable. Upon examining the total counts (all race/ethnic groups included) in Table 11-3, it appears that there is a small decrease in the total number of students from 38,744 (2003-2004) to 36,710 (2007-2008), with a peak in 2005-2006 with 39,502 students.
TABLE 11-3 Number and Percentage of Students with Hearing Impairments by Race/Ethnicity during the 2003 to 2008 School Years*
The GRI Annual Survey provided information regarding the children’s home environment. For each of the 5 years, data indicated that the majority of students had “hearing” parents. On an average, the mother’s hearing status was reported to be hearing for 91.08% of the students, deaf for 4.4% of the students, and hard of hearing for 2.2% of the students. The father was reported to be hearing on an average for 85.18% of the students, deaf for 4.66% of the students, and hard of hearing for 2% of the students. Both parents were reported to be hearing for 82.14% students, and deaf or hard of hearing for 3.94% of the students. When asked about the use of sign language in the home, it was reported that a majority (70.3%) of family members did not regularly use sign language at home. Only an average of 25.34% of family members signed at home. Data on the languages spoken at home were available only for years 2006-2007 and 2007-2008. The vast majority (average = 82.85%) used English to communicate at home, whereas a much smaller number (average = 21.5%) reported that they spoke Spanish at home. Numerous other languages were reported to be used at home, but those figures were miniscule.
Epidemiology of hearing loss and deafness in other countries in the world
Southeast asia
Schmitz and colleagues (2010) conducted a study to assess the prevalence and severity of hearing loss and “middle ear dysfunction” in a group of 15- to 23-year-olds in a rural area of Southern Nepal. They reported that 6.1% of the subjects had hearing loss. Further, they reported that persons who were hearing impaired were six to eight times more likely to report problems in hearing sounds of daily living and in communicating with others.
China
Wong and McPherson (2008) reported that the incidence of hearing loss in China is probably higher than the 21 million reported by the National Bureau of Statistics. More than 14 million persons in China, who are older than 60 years, have hearing loss. If only those older than 65 years are considered, the incidence is higher and growing at a rate of 3% to 4% annually. Sun and associates (2008) examined data from the Second China National Sample Survey on Disability and reported that 27.80 million persons were diagnosed with hearing loss, which was an overall prevalence rate of 2.11%, although the incidence was higher (11.04%) among elderly persons. The prevalence was higher among males than females and higher in rural areas compared with urban areas. Fu and coworkers (2010) investigated the prevalence of hearing loss in students enrolled in primary and middle schools in the Hubei province of China. During their 2-year study, 504,348 students were tested; 813 were deaf of whom 232 children were diagnosed with congenital deafness, and 560 had acquired deafness. Of the latter, 276 had aminoglycoside antibiotic–induced deafness. The severity of deafness in 804 other students was distributed as follows: profound (402), severe (363), moderate (21), and mild (18). The age of onset for most children was under 3 years of age. The prevalence rates of congenital and acquired deafness were 0.046% and 0.111%, respectively. Liu and associates (2001) conducted a large-scale epidemiological survey of 126,876 persons (63,741 male, 63,135 female) in Sichuan, China. They reported an overall prevalence of 3.28% for hearing loss, which increased to 12.8% at 60 years of age; 73.03% of all cases had sensorineural hearing loss, whereas 20.39% had conductive, and the remaining 6% had mixed hearing loss. They also reported that 74.5% of the cases had bilateral hearing loss, Further, 63.79% of cases had mild to moderate hearing loss (<55 dB HL), and 5.67% had profound hearing loss (>90 db HL). In children younger than 15 years, the prevalence of hearing loss was 0.67%; 57.7% of the cases had conductive hearing loss, and 38.8 had sensorineural hearing loss. They reported that persons who lived in the flatlands appeared to have a higher prevalence of hearing loss than those who lived in the hills. Further, they reported that several ethnic groups, namely, Tibetans, the Yi, and the Lisu, had a higher prevalence of hearing loss.
The middle east
Attias and associates (2006) ascertained the prevalence of congenital and early-onset hearing loss in infants in Jordan and Israel using Distortion Product Otoacoustic Emissions (DPOAE) for screening hearing, with follow-up using Auditory Brainstem Response (ABR) when needed. They found that the prevalence of hearing loss in infants in Jordan (1.37%) was significantly higher than in the infants in Israel (0.48%). Further, the prevalence of bilateral sensorineural hearing loss was seven times higher in the infants in Jordan than in the infants in Israel. Also, in the Israeli sample, the prevalence of hearing loss in the no-risk infants was 0.19%, compared with 8% (42 times greater) in the high-risk infants. In the Jordanian sample, the prevalence was 0.51% in the no-risk infants and 10% (19 times greater) in the high-risk infants. The overall prevalence of hearing loss in the Jordanian sample was remarkably higher (about 2.8 times) than that in the Israeli sample. Risk factors, such as family history, hyperbilirubinemia, bacterial meningitis, and associated syndromes, were more prevalent in infants in Jordan.
Al Khabori and Khandekar (2004) used pure tone audiometry to screen the hearing of 12,400 persons of all ages in Oman. Children younger than 4 years were tested by subjective methods, involving a set of questions and observations. The prevalence of bilateral hearing loss in Oman was 55 per 1000 persons. The prevalence of bilateral hearing loss was highest in persons who were 60 years or older (325 per 1000), and lower (17 per 1000) in children younger than 10 years.
Abdel-Hamid and coworkers (2007) used DPOAE and impedance audiometry to screen 4000 individuals for hearing loss from six randomly selected governorates in Egypt. The prevalence of hearing loss was 16%, but there were differences between age groups and governorates. Marsa Matrrough, a governorate, had the highest prevalence of hearing loss (25.7%), whereas North Sinai had the lowest percentage (13.5%). Persons who were 65 years and older had the highest prevalence (49.3%), followed by children 0 to 4 years of age (22.4%).
Australia
Currently, one in six persons in Australia has a hearing loss, but it is estimated that this figure will rise to one in four by 2050 as the population ages. Approximately 9 to 12 children per 10,000 live births in Australia are born with a bilateral moderate or greater hearing loss, whereas an additional 23 children per 10,000 acquire a hearing impairment by age 17 years that necessitates the use of hearing aids. By the age of 60 to 70 years, more than 50% of Australians have a hearing loss; this figure increases to 70% or more among those who are older than 70 years and to more than 80% for persons older than 80 years. Men have a higher incidence of hearing loss than women, primarily related to exposure to occupational noise, which is the primary cause of hearing loss in adults in Australia. For instance, more than 50% of farmers have hearing loss because of exposure to noise from agricultural machinery. Hearing loss costs Australia approximately $12 billion annually (Australian Hearing, n.d.; Williams, 2010).
Quinn and Rance (2009) investigated the hearing of 109 indigenous prisoners at five prison locations in Victoria, Australia. Primarily the prisoners had mild sensorineural hearing loss. The rate of conductive hearing loss was consistent with that found in an age-matched adult general population in the United Kingdom. Twelve percent of prisoners had a hearing loss of 25 dB or greater in at least one ear, compared with the incidence of 5% in the general adult Australian population. Thirty-six percent of the inmates had unilateral or bilateral high frequency sensorineural hearing loss; 58% reported having hearing problems some of the time, and 4% reported having a lot of trouble in hearing. Ninety-two percent of inmates reported having had exposure to loud noise; 72% reported having tinnitus.
Russ and colleagues (2003) investigated the incidence and clinical characteristics of congenital hearing loss that was sufficient to necessitate fitting hearing aids during the first 6 years of life in children born in 1993 in Victoria, Australia. Only 2.09 per 1000 children had been fitted with hearing aids. Of these, 54 children (40%) had mild hearing loss (20 to 40 dB HL). The researchers reported that the prevalence of moderate or greater loss (>40 dB HL) was 1.12 per 1000 children, that of severe or greater loss (>60 dB HL) was 0.48 per 1000 children, and that of profound hearing loss (>90 dB HL) was 0.17 per 1000 children.
Denmark and the netherlands
Dammeyer (2010) reported the prevalence of “deaf blindness” in Denmark to be 1:29,000. Meuwese-Jongejeugd and associates (2006) reported on the prevalence of hearing loss in 1598 adults with an intellectual disability (ID) in the Netherlands. The researchers reported a prevalence ranging from 7.5% in a subgroup aged 18 to 30 years, who did not have Down syndrome but did present an ID, to a prevalence of 100% in adults older than 60 years with Down syndrome. The researchers concluded that in persons with Down syndrome, age-related hearing loss occurs approximately three decades earlier than in the general population. Similarly, in persons who have an ID due to causes other than Down syndrome, hearing loss occurs approximately one decade earlier than in the general population.
Canada
The Participation and Activity Limitation Survey (PALS) is a post-censal national survey funded by Human Resources and Social Development Canada (HRSDC) and conducted by Statistics Canada (2009). It is designed to collect information on adults and children who have an activity limitation because of a health problem. Statistics Canada (2001) reported that 23,750 children aged 0 to 14 years had hearing loss, of whom 14,230 were males and 9520 were females. Of the 23,750 children, 3160 children were 0 to 4 years of age, 10,800 were 5 to 9 years of age, and 9790 were 10 to 14 years of age. Brennan and coworkers (2006) reported that 1,266,120 Canadians aged 15 years and older reported having a hearing limitation on the 2006 PALS. The majority (60.8%) of persons with hearing difficulty reported having some hearing loss. More than 8 of 10 (83.2%) hearing limitations were mild in nature, and 16.8% were classified as severe.
Millar (2005) reported the results of the 2003 Canadian Community Health Survey (CCHS), which is a general survey used to collect information on persons 12 years and older; it precludes those on Indian reservations, Canadian forces bases, and some remote areas. Three percent of the Canadian population aged 12 years and older had some difficulty with their hearing. The incidence increased with age. Accordingly, although seniors made up only 14% of the 12 years or older population, they represented 55% of persons with hearing problems. Of those who were 65 years or older, approximately 11% had a hearing problem. Of those who were 65 to 69 years old, 5% had a hearing problem, and among those who were 80 years and older, 23% had a hearing problem. The percentage of persons reporting a hearing problem by province from the lowest incidence to the highest are as follows: Québec (7%), Ontario (10%), Manitoba (11%), Alberta(13%), Nova Scotia (14%), Prince Edward Island, Newfoundland, New Brunswick, British Columbia (15% each), and Saskatchewan (16%).
Early identification of hearing loss
Early identification of hearing loss in the united states
Undetected permanent bilateral hearing loss in children can lead to significant delays in the development of language, cognition, speech, and literacy, which in turn affects education and increases the burden on society. For instance, the CDC (2004) estimated that the lifetime costs for all people with hearing loss in the United States, who were born in 2000, would total $2.1 billion. During the 1999-2000 school year, the total cost for special education programs for children who were deaf or hard of hearing in the United States was $652 million, or $11,006 per child (Chambers, Shkolnik, & Perez, 2003). The lifetime educational cost of hearing loss of more than 40 dB HL was estimated to be approximately $115,600 per child (Grosse, 2007).
Interest in infant hearing screening dates back to the mid-1940s when behavioral responses to auditory stimuli were observed. Continued interest in infant hearing screening led to the formation of the Joint Committee on Infant Hearing (JCIH). Healthy People, 2000 included the goal to reduce the average age at which children with significant hearing loss were identified to no more than 12 months of age (U.S. Department of Health and Human Services, 1990). Dramatic advances in technology in the 1990s led to the invention of more sophisticated audiology equipment and automatic hearing screeners. An expert panel convened by the National Institutes on Deafness and Other Communication Disorders (NIDCD) in 1993 concluded that all infants admitted to NICUs should be screened before discharge, and universal hearing screening should be implemented for all infants within the first 3 months of life. The JCIH (2000) endorsed the development of a system for detection and intervention that was family centered and community based and emphasized the need to identify children with permanent sensory or conductive hearing loss averaging 30 to 40 dB HL or more in the frequency region that is important for speech recognition (i.e., 500 to 4000 Hz).
Goal 28-11 of Healthy People 2010 (U.S. Department of Health and Human Services, 2001) required an increase in the proportion of newborns screened for hearing loss by 1 month of age, required audiologic evaluation by 3 months, and required provision of appropriate intervention services by 6 months of age. The number of infants screened for hearing loss in the United States increased dramatically from 46.5% in 1996 to 97% in 2007. Further, in 2007, 83% of babies with diagnosed hearing loss were referred to Part C Early Identification Services. To identify babies with hearing loss, all states and territories of the United States have established early hearing detection and intervention (EHDI) programs, which ensure that all newborns and infants are screened and receive recommended follow-up through data collection and outreach to hospitals, providers, and families. Although the high percentage of infants lost to follow-up or lost to documentation continues to be a major challenge for EHDI programs, substantial progress has been made toward achieving benchmarks for screening, evaluation, and intervention (CDC, 2010d; 2011). In 2009, 97% of newborns were screened for hearing loss. Of these, 1.6% did not pass the final or most recent screening. Of those who did not pass the screening 67.9% were diagnosed as having or not having a hearing loss by 3 months of age. The current prevalence rate of newborns with hearing loss is approximately 1.4 per 1000 babies (CDC, 2011).
Early identification of hearing loss in other countries in the world
The netherlands
Unlike many European countries and the United States, in the Netherlands, approximately 35% of babies are born at home, and about 85% of those born in hospitals leave within 24 hours of birth. Sixty-five percent of home-based and hospital deliveries are attended by midwives. The Netherlands has had nationwide screening for hearing loss since the 1960s, but was using distraction testing. Although 96% of babies were tested during the first year of life, over the years, there was growing dissatisfaction with the program because of the high and increasing false-positive rate primarily due to conductive hearing loss. The 5% referral rate in the early 1980s grew to 7% within a few years. Additionally, there was concern because children were diagnosed with permanent hearing loss at about 18 months of age or later. Several studies were conducted to ascertain whether universal neonatal hearing screening could be integrated in the youth health care program in the Netherlands, which provides services that include vaccinations; monitoring of cognitive, motor, and language development; and screening for metabolic disorders, visual and hearing problems to children from birth through 19 years. In one such study, the screening was performed by nurses of well-baby clinics on 3114 newborns. Three-stage transient otoacoustic screening was administered in three different screening settings; in one setting parents visited well-baby clinics, and in two other settings the screening was done at home. The researchers determined that the most efficient and effective setting for universal hearing screening was where hearing screening was integrated with screening for metabolic disorders. The participation rate was the highest (89.9%) and the overall referral rate the lowest (1.4%) in this setting. The results of this study formed the basis for nationwide implementation of universal hearing screening in that country (Uilenburg et al., 2009).
Between 2002 and 2006, all 65 regions in the Netherlands replaced distraction hearing screening with newborn hearing screening. During the transition, some regions switched to newborn hearing screening first, whereas other regions continued to use distraction hearing screening. To ascertain whether this change affected developmental outcomes in children with permanent childhood hearing loss, Korver and coworkers (2010) compared the effect of newborn hearing screening and distraction hearing screening conducted at 9 months of age on development, spoken communication, and quality of life in children. All children born in the Netherlands from 2003 to 2005 were included in the study; thus, 335,560 children in newborn hearing screening regions and 234,826 children born in distraction hearing screening regions participated in the study. At follow-up, 263 (0.78 per 1000) children in newborn hearing screening regions and 171 (0.73 per 1000) in the distraction hearing screening regions had been diagnosed with permanent childhood hearing impairment. All children with permanent hearing impairment were identified by 3 to 5 years of age. Of these, 301 (69.4%) children participated in the analysis of the general performance measures. The two groups did not differ in terms of the primary mode of communication or type of education. However, children in the newborn hearing screening regions had higher overall scores for social development, gross motor development, and quality of life measures than children in the distraction hearing screening regions.
Germany
In 2007, the German Society for Phoniatrics and Pediatric Audiology developed a quality assurance program for universal newborn hearing screening (Neumann et al., 2009). The recommendations included the procedures for screening and subsequent follow-up as well as criteria and definitions of levels of hearing loss.
Rohlfs and associates (2010) described a regional interdisciplinary universal hearing screening program started in Hamburg, Germany, in 2002. Reportedly, this was the first time that a comprehensive protocol that included screening, follow-up procedures, tracking, and early intervention was implemented in Germany. Of 65,466 births registered between 2002 and 2006, 63,459 (93%) newborns were screened; 3.3% failed screening, and 31.3% were lost to follow-up. A total of 118 children were diagnosed with hearing loss at a median age of 3.5 months. Seventy-four children were fitted with hearing aids, of whom 6 were subsequently implanted. Rohlfs and associates reported that their biggest challenge was the high percentage of children lost to follow-up.
Italy
Italy has shown considerable growth in implementing universal new born hearing screening (UNHS). Bubbico and colleagues (2008) reported on the degree of coverage of UNHS in Italy. The number of infants screened for hearing loss increased from 156,048 (29.3%) in 2003 to 262,103 (48.4%) in 2006. The researchers reported that the majority of UNHS programs have been implemented in the most economically developed areas; 108,200 (79.5%) of newborns were screened in the northwest, and 92,133 (57.2%) newborns were screened in the northeast. However, because there are fewer UNHS programs in the Italian islands, including Sardinia and Sicily, only 7158 (11.3%) of newborns were screened in the islands.
Canada
Durieux-Smith and coworkers (2008) reported on the ages of diagnosis and hearing aid fitting of 709 Canadian children with congenital or early-onset permanent hearing loss who were identified between 1980 and 2003 through neonatal hearing screening programs or through medical referrals. Analysis showed that children who were screened were diagnosed earlier, at a mean age of 6.3 months, compared with children who were referred and diagnosed at a mean age of 39.5 months. The researchers reported that over the years, the age at which referred children, were diagnosed and fitted with hearing aids improved but still remained unacceptably high. Further, it was observed that children with lesser degrees of hearing loss were diagnosed at older ages than those with severe to profound hearing loss. The results of this study lend strong support to the benefits of implementing a newborn hearing screening program, diagnosis at an early age and possibly early hearing aid fitting, which greatly facilitates the development of language, speech, cognitive and literacy skills.
Developing countries
In developing countries, hearing loss remains a silent epidemic. Although developed countries, such as the United States and United Kingdom, have legislated the implementation of universal hearing screening programs, unfortunately, such programs for early detection of hearing loss are not well established or not established at all in the developing countries where the incidence of hearing loss is extremely high and the human and financial resources are meager at best (Olusanya, 2006, 2008; Theunissen & Swanepoel, 2008). Although universal hearing screening has been hospital based in most developed countries, it may not be a feasible option in many developing countries where a majority of births occur at home or in birthing facilities.
Olusanya and associates (2008) indicated that hospital-based universal hearing screening programs were unlikely to be effective in Lagos, Nigeria because the majority of births did not occur in hospitals. On the other hand, routine childhood immunization programs often provided an effective community-based avenue for attracting babies born outside of hospitals. To ascertain the effectiveness of such an arrangement, they set up a screening during bacilli Calmette-Guérin (BCG) immunization (first vaccination given typically within the first month of life to Nigerian babies) in an inner-city area at four health care centers that accounted for 75% of BCG vaccinations. They used a two-stage screening protocol; transient evoked otoacoustic emissions (TEOAE) was administered initially, followed by ABR. They observed an overall prevalence rate of 30.7 per 1000, whereas 19.2 per 1000 had moderate to severe hearing loss. Significantly more males than females were found to have hearing loss. The researchers concluded that permanent hearing loss was a highly prevalent disability in Nigeria; it could be detected early, and community health workers at primary care centers could be effectively used for universal hearing screening.
In Benin City, Nigeria, Okhakhu and coworkers (2010) screened 400 neonates using an OAE screener. Ninety neonates (22.5%) failed the screening and were referred for further testing. Of the 90 neonates, 26 (6.5%) were found to have bilateral hearing loss, and 64 (16%) were found to have unilateral hearing loss. Okhakhu and coworkers concluded that neonatal screening was necessary in all developing countries. Similarly, Swanepoel and colleagues (2007) reported on an infant hearing screening program that was developed using immunization clinics in South Africa as part of the primary, secondary, and tertiary levels of health care.
Lin and associates (2002) assessed the feasibility and cost-effectiveness of implementing universal newborn hearing screening in Taiwan. They screened 6765 newborns before discharge from a hospital using TEOAE. The overall pass rate was 93.6%; 6.4% of newborns were referred for diagnostic audiological assessments. Nine newborns were diagnosed to have permanent bilateral hearing impairment, and 26 newborns were identified to have permanent unilateral hearing loss. The newborns with bilateral hearing loss were referred for hearing aids. The researchers concluded that in Taiwan, universal newborn hearing screening using TEOAE was practicable and cost-effective.
Taiwan’s health insurance system apparently does not facilitate newborn hearing screening. From March 2000 through December 2002, Lin and associates (2004) piloted a community-based pay-for-test (parents paid for the TEOAE test) newborn hearing screening program in two hospitals and four obstetric clinics. A total of 5938 healthy newborns were tested in newborn nurseries before discharge using TEOAE. Of these, 5403 (91%) newborns passed the screening, whereas 535 (9%) failed it. One hundred and forty babies were lost to 1-month follow-up. However, 395 (73.8%) infants underwent a second test at the outpatient clinic, of whom 91 failed and were referred for ABR testing. Ultimately, 9 babies were diagnosed with sensorineural hearing loss. The researchers concluded that a pay-for-test model was feasible in Taiwan, was well accepted by parents, and could be implemented without financial support from the government.
Bevilacqua and colleagues (2010) developed a newborn hearing screening program in a public hospital in Brazil. 11,466 newborns were screened using TEOAE and a 2-stage approach. The researchers reported that the prevalence rate of sensorineural hearing loss was 0.96 per 1000 newborns. Eight of 11 children were fitted with hearing aids and 4 children were implanted.
Etiology
Deafness or hearing loss at birth or in early childhood can have a devastating effect on a child’s language, speech, and cognitive development, which in turn negatively affects the child’s emotional and educational experiences. As the child grows older, there are social, vocational, and economic consequences that reduce the quality of life. Advances in genetic technology have greatly facilitated identification of the etiology of hearing loss in many cases, which in turn may facilitate management and may provide the information parents want. Harrison and Roush (2002) asked parents what information they wanted to receive pertaining to their child’s hearing loss at the time of the initial diagnosis. Parents reported that they wanted to know the cause of their child’s hearing loss. Some even reported that the uncertainty of not knowing the cause made it difficult for them to focus on intervention issues.
Morzaria and colleagues (2004) ascertained the frequency of etiologies of moderate to profound bilateral sensorineural hearing loss in children. They found that the most common causes of bilateral sensorineural hearing loss are unknown (37.7%), genetic nonsyndromic (29.2%), prenatal (12%), perinatal (9.6%), postnatal (8.2%), and genetic syndromes (3.2%). Wormald and associates (2010) examined the etiology of sensorineural hearing loss in children presenting at a medical center in Ireland. They reported that 66% of children had hereditary hearing loss, 8% had acquired hearing loss, and the etiology was unknown in 26% of children. Data from the Gallaudet Research Center’s Annual Survey of Deaf and Hard of Hearing Children and Youth for the years 2003-2006 indicated that excluding otitis media, the most prevalent etiological factors consistently reported each year on the survey are genetic/hereditary/familial, prematurity, complications of pregnancy, other postbirth causes, and CMV.
Low birth weight
Some races and ethnic groups are disproportionately affected by the high infant mortality rate for low-birth-weight infants. Matthews and MacDorman (2010) reported rates that ranged from 4.52 per 1000 live births for Central and South American mothers to 13.35% for non-Hispanic black mothers. Other factors associated with low birth weight in neonates include maternal age (e.g., teenage pregnancies), delayed or no prenatal care, mother’s disadvantaged background, maternal education, maternal use of tobacco, and so forth. According to the JCIH (2000), infants weighing less than 1500 grams are at risk for hearing impairment or deafness. Therefore, it may be inferred that more African American babies are at risk for hearing impairment or deafness than other ethnic groups.
Neonatal intensive care units (NICUs) are known to reduce mortality among infants with low birth weight. Barfield and coworkers (2010) analyzed birth data from 19 states to ascertain the prevalence of admission of infants with very low birth weight (VLBW) to NICUs. The rate of admission varied by race and ethnicity among states: a smaller percentage (71.8%) of infants with VLBW born to Hispanic mothers were admitted to NICUs, compared with infants born to non-Hispanic black mothers (79.5%) and infants born to non-Hispanic white mothers (80.5%). The researchers reported that greater prevalence of admission of infants with VLBW to NICUs was associated with three factors: preterm delivery, multiple births, and cesarean delivery.
Coenraad and colleagues (2010) evaluated etiological factors associated with sensorineural hearing loss in infants who were admitted to NICUs in the Netherlands. Normal hearing controls were used for comparison. Of the 3316 infants screened, 58 infants (26 girls, 32 boys) were diagnosed with sensorineural hearing loss. Although numerous risk factors were examined, the incidence of dysmorphic features, low Apgar score (1 minute), sepsis, meningitis, cerebral bleeding, and cerebral infarction were significantly increased in infants with sensorineural hearing loss compared with the normal hearing controls.
After conducting a 30-year longitudinal study in Canada, Robertson and associates (2009) reported that permanent hearing loss is an adverse outcome of extreme prematurity. This hearing loss may have a delayed onset and may be progressive. Their subjects had a gestational age of 28 weeks or less and birth weight of less than 1250 grams. They reported that prolonged supplemental oxygen use was a marker for predicting permanent hearing loss. Tomasik (2008) also examined risk factors for hearing impairment in 218 prematurely born Polish infants with birth weights of 520 to 3000 grams and gestational age of 22 to 36 weeks. Tomasik concluded that the following factors put premature infants at risk for hearing loss: low gestational age or associated with it prolonged mechanical ventilation, hyperbilirubinemia, severe general condition, hypoglycemia, and prolonged treatment with amikacin.
Xoinis and coworkers (2007) conducted a retrospective study on NICU graduates to establish a prevalence rate for auditory neuropathy. They reported a prevalence of sensorineural hearing loss in 16.7 per 1000 NICU infants and a prevalence of auditory neuropathy in 5.6 per 1000 infants. They reported that compared with infants with sensorineural hearing loss, infants with auditory neuropathy had younger gestational ages and lower birth weights. In fact, two thirds of infants with auditory neuropathy were infants with extremely low birth weight and had significantly longer hospital stays compared with infants with sensorineural hearing loss.
To examine the impact of birth weight on risk for sensorineural hearing loss in Norwegian children, Engdahl and Eskild (2007) compared children with sensorineural hearing loss with those in a control group. They observed that the risk for hearing loss decreased with increasing birth weights. They concluded that the risk for both mild to moderate and severe to profound sensorineural hearing losses were influenced by birth weight. Martinez-Cruz and colleagues (2008) investigated risk factors associated with sensorineural hearing loss in infants in a tertiary level NICU and a control group in Mexico City. They reported that low birth weight, longer stay in a NICU under mechanical ventilation, higher serum bilirubin levels, blood transfusion, intraventricular hemorrhage, and meningitis were the main risk factors associated with sensorineural hearing loss.
Olusanya (2011) studied undernourished infants in sub-Saharan Africa to determine risk factors for early-onset permanent hearing loss. Of 2254 infants, 39 (1.7%) were confirmed to have hearing loss, of whom 7 had mild hearing loss and 26 had moderate to profound hearing loss. “Absence of skilled attendant at birth and severe neonatal jaundice” were among the factors associated with the permanent hearing loss.
Genetic hearing loss
Epidemiologic studies in the United States have shown that approximately 1 in 1000 to 2000 children are born with or present in early childhood with severe or profound hearing impairment, of whom approximately half have a genetic cause (Parving, 1983; Newton, 1985). Marazita and colleagues (1993) reported that the incidence of profound early-onset deafness was present in 4 to 11 per 10,000 children. Of these, 37.2% of the cases were attributed to sporadic causes and 62.8% to genetic causes (47.1%, recessive; 15.7% dominant). Recent studies have suggested that approximately 50% of moderate to profound, congenital, or early-onset hearing loss is genetic in origin. A genetic hearing impairment may occur in isolation (nonsyndromal) or in association with other features (syndromal). Nonsyndromal hearing impairments (70%) occur as a single gene disorder due to an autosomal dominant gene, X-linked gene, or mitochondrial inheritance. For instance, adult-onset progressive hearing impairment can be caused by mutations in a single gene. Syndromal hearing impairments (30%) result from chromosomal or single gene causes. A syndrome is defined as multiple anomalies in a person, with all of the anomalies having a single cause, which may be a genetic mutation, chromosomal abnormality, teratogen, or some extrinsic factor. There are more than 400 known syndromes with hearing loss as one of the anomalies. In the case of a syndrome, hearing loss may be just one of several problems. A few of the syndromes associated with hearing loss are Usher syndrome, Pendred syndrome, and Turner syndrome.
Data from the Annual Survey of Deaf and Hard of Hearing Children and Youth for the years 2003 to 2007 conducted by the Gallaudet Research Institute consistently showed that genetic/hereditary/familial factors accounted for the largest percentage of hearing impairment/deafness than any other etiological factor. The incidence ranged from 21.8% in 2003-2004 to 23.6% in 2007-2008. During the years 2004-2005, 2005-2006, and 2006-2007 the incidence rates were 22.7%, 23%, and 23.3% respectively (Gallaudet Research Institute, January 2005; December 2005; December 2006; December 2007; November 2008).
Based on data from the 1989-1990 Annual Survey of Hearing Impaired Children and Youth (Nuru, 1993) reported that the incidence of genetic hearing loss varied across races and ethnic groups. Similarly, the 1991-1992 Annual Survey of Hearing Impaired Children and Youth (Schildroth, 1994) indicated that heredity as a causative factor was the highest among white (72%) children and youth when compared with peers from the Hispanic (14%), African American (10%), Asian and Pacific Islander (2%), and other (2%) communities.
Parving (1996) summarized the incidence of hearing impairment in children that was attributed to genetic causes based on 14 surveys conducted internationally. The incidence of genetic hearing impairment reported in these surveys varied from 9% to 54%, whereas 16% to 42% of the children had hearing impairment of unknown origin. Parving suggested that it was likely that the variation in the proportion of genetic hearing impairment reflected true differences in the genetic expression in the different populations. In the United States, autosomal recessive nonsyndromal hearing impairment is estimated to occur in 1 of 1000 children (Morton, 1991), whereas in England and Denmark, it is reported to occur in 0.7 of 1000 children (Davis & Parving, 1994). However, in Sichuan, China, it was estimated that the prevalence rate of genetic hearing loss was 0.28% (Liu et al, 2001).
Cultural practice in a community affects the incidence of genetic hearing impairment. In some cultures, consanguineous marriages are an accepted practice. Al-Shihabi (1994) reported that the incidence of hearing impairment was 12.9 per 1000 in offspring in consanguineous marriages but 3.1 per 1000 births in nonconsanguineous marriages in the same geographic area. This suggested that autosomal recessive hearing impairment was a monogenic disease. The Sultanate of Oman in the Arabian Peninsula is still predominantly tribal. Al Khabori and Patton (2008) reported on a retrospective analysis of 1400 questionnaires (which the ear-nose-throat [ENT] staff completed at the time of mandatory hearing screening administered when a child first started school) on the etiology of deafness in Omani children collected between 1986 and 2000. They observed that 70% of children with deafness were from parents of consanguineous marriages. Among consanguineous families, 70.16% were first-cousin marriages, 17.54% were second-cousin marriages, and 10.86% were from the same tribe. In addition, 45% of the total cohort had other family members with hearing loss. There was a greater chance of other relatives having a hearing loss in the consanguineous group (29.7%) than in the nonconsanguineous group (15.3%). In most cases, the affected relative was a sibling with deafness (67.8%) indicating a high frequency autosomal recessive transmission.
Mueller (2000) reported that investigations of the recurrence of hearing impairment in children born to hearing-impaired or deaf couples have indicated that there are approximately 100 genes that may be responsible for nonsyndromal sensorineural hearing impairment or deafness. During the past 8 years, 28 autosomal recessive, 30 autosomal dominant, and 5 X-linked genes for nonsyndromal sensorineural hearing impairment or deafness have been cloned. The gene known as GJB2 (Gap Junction Beta 2), or Connexin 26 or CX26, which is located on chromosome 13, accounts for approximately 50% of nonsyndromic sensorineural hearing loss. GJB2 produces a protein called connexin 26, which creates channels between cells through which small molecules diffuse. It has been suggested that this protein may be involved in maintaining potassium levels in the cochlea. The hearing loss associated with mutations in GJB2 varies in severity, generally ranging from moderate to profound, although most range from severe to profound hearing loss. GJB2 mutations were identified first in 1997. Since then, more than 90 GJB2 mutations have been reported. It is now known that three specific mutations (35delG, 167delT, 235delC) are responsible for the majority of GJB2-related hearing loss. 35delG is more prevalent in whites of Northern European descent as well as persons from the Mediterranean and Middle Eastern regions. 167delT is found in the Ashkenazi Jewish population, and 235delC is observed primarily in Asian populations (Palmer et al., 2003). By testing for this gene, it is possible to identify the cause of deafness in 20% to 40% of persons with hearing impairment or deafness of unknown etiology. For instance, Morell and colleagues (1998) reported that the 167delT mutation had only been identified in the Ashkenazi Jewish population. On the other hand, the 35delG mutation in Connexin 26 has been shown to account for nonsyndromal sensorineural hearing impairment or deafness in Western Europe and North America (Estivill et al., 1999; Lench et al., 1998; Zelante et al., 1997). To examine the influence of 35delG on autosomal recessive nonsyndromic hearing loss, Mahdieh and Rabbani (2009) conducted a meta-analytical review. They reported mean carrier frequency for this gene to be 1.89 for Europeans, 1.52 for Americans, 0.64 for Asians, 1 for Oceania, and 0.64 for African populations. Further, they reported that the carrier frequency was highest in southern Europe and lowest in eastern Asia.
Researchers from around the globe have been investigating the role of GJB2 in hearing loss in their countries and communities. Löppönen and associates (2003) examined Connexin 26 mutations and nonsyndromic hearing impairments in northern Finland, and observed that 21.1% of the hearing impaired children they examined had CX26 mutations. Mutation 35delG was the cause of hearing impairment in 86.7% of the children. The carrier frequency for the mutation 35delG was 1 of 78, whereas that for mutation M34T was 1 of 26. Batissoco and associates (2009) determined the frequencies of GJB2 mutations and GJB6 deletions in the Brazilian population. They screened 300 persons with hearing impairment, who did not have known deafness syndromes. The most frequently found mutation, 35delG, was seen in 23% of familial and 6.2% of sporadic cases. The second most frequently occurring mutation was a deletion in the GJB6 gene (GJB6-D13S1830).
Propst and coworkers (2006) examined the relationship between ethnicity and mutations in the GJB2 and GJB6 genes in multicultural patients enrolled in a pediatric cochlear implant program in Canada. Nine different GJB2 mutations were identified in persons from 14 different countries. About 78% of all identified pathogenic GJB2 mutations were 35delG. The researchers observed that individuals of African, Caribbean, and East Indian descent had different GJB2 mutations compared with other individuals who were tested. Gravina and associates (2010) examined the prevalence of GJB2 mutations and GJB6 deletions in Argentinean children with nonsyndromic deafness. They observed that the most frequently found GJB2 mutation was 35delG, and the second most common mutation was deletion GJB6-D13S1830. The frequency of the deletion was as high as that “found in Spain from where Argentina has received one of its major immigration waves.” Teek and colleagues (2010) studied the prevalence of 35delG and M34T mutations in the GJB2 gene in children with early-onset hearing loss as well as within the general population of Estonia. Based on their data, they concluded that in Estonian children with early-onset hearing loss, the most common GJB2 mutations were 35delG and M34T. In the general population, 1 of 22 persons carried the 35delG mutation, and 1 of 17 carried the M34T mutation. Siem and associates (2010) reported that GJB2 mutations were a common cause of hearing impairment in Norwegian children with cochlear implants; 35delG mutation accounted for 85% of mutations. Similarly, Cama and colleagues (2009) found that 35delG was one of the most frequent (64%) mutations in their patients from the Veneto region in Italy.
Unlike the European researchers, investigators from China and Japan found the 235delC mutation to be the most prevalent. Chen and coworkers (2009) enrolled 115 cochlear implant patients for mutation screening to ascertain whether GJB2 mutations were the major causes of deafness in Chinese cochlear implant recipients. They reported that 36.5% of their cochlear implant recipients and 41% of nonsyndromic deafness patients had GJB2 mutations. They found 11 variations in the GJB2 gene; the 235delC mutation was the most prevalent mutation. Similarly, Hayashi and associates (2011) conducted mutation screening and direct sequencing for GJB2 in 126 Japanese children who had been implanted. They also observed 10 different mutations, but the one that was most prevalent (44.8%) was the 235delC mutation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







