Chapter 15 Multiple Sclerosis
Clinical Manifestations
Course
Almost all MS patients follow one of four reasonably distinct courses, disease categories, that consist of multiple attacks, steady deterioration, or several attacks followed by steady deterioration (Fig. 15-1, top). The categories reflect the clinical status as it relates to time. They do not take into account the severity or results of MRIs.
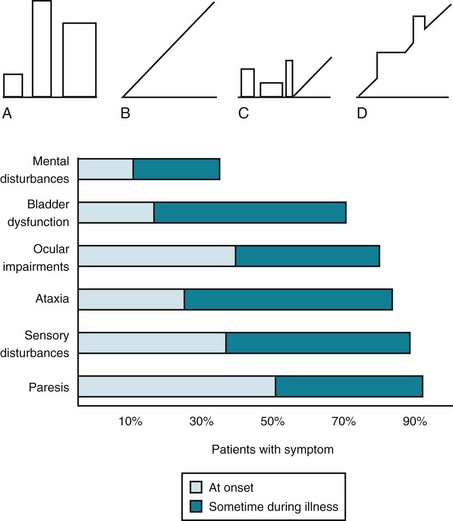
FIGURE 15-1 Top, Graphs of different clinical courses – with severity of multiple sclerosis (MS) attacks (vertical axis) plotted against time (horizontal axis) – reveal four patterns or disease categories: A, Relapsing-remitting; B, primary progressive; C, secondary progressive; D, progressive-relapsing. Bottom, This chart of initial and cumulative manifestations of MS indicates that cognitive impairment develops infrequently at the onset and ultimately less often than physical impairments.
Frequent Symptoms
Lesions in the white-matter tracts of the CNS cause various symptoms during the course of a patient’s illness (Fig. 15-1, bottom). Moreover, simultaneous involvement of two separate CNS areas often produces combinations of disparate symptoms. For example, plaques may simultaneously develop in the cerebellum and thoracic spinal cord, which would cause ataxia and paraparesis.
Cerebellar Signs
As some of their earliest manifestations, MS patients often develop ataxia, intention tremor, and other signs of cerebellar and cerebellar outflow tract injury. When the cerebellum is involved, patients typically develop an ataxic gait (see Fig. 2-13); however, with minimal involvement, patients’ gait impairment may consist only of difficulty walking in a heel-to-toe (tandem gait) pattern. Cerebellar involvement also typically causes scanning speech, a variety of dysarthria analogous to a “speech ataxia,” characterized by irregular cadence and uneven emphasis on words. For example, when asked to repeat a pair of short syllables, such as “ba…ga…ba…ga…,” a patient might place unequal stress on different syllables, blur them together, or pause excessively. Other manifestations of cerebellar involvement include intention tremor (see Fig. 2-11), dysdiadochokinesia, and an irregular, conspicuous, head tremor (titubation).
Decreased Visual Acuity
Neurologists usually attribute visual acuity impairment in MS to inflammation in the retrobulbar portion of the optic nerve, retrobulbar neuritis (optic neuritis) (see Fig. 12-6). Optic neuritis typically causes an irregular area of visual loss in one eye, a scotoma, that often includes the center of vision (Fig. 15-2). It also leads to color desaturation, in which colors, especially red, lose their intensity.
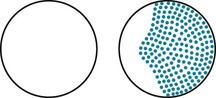
FIGURE 15-2 Optic or retrobulbar neuritis impairs vision in a large, irregular area (scotoma) of the affected eye.
Optic neuritis, as well as other lesions of the optic nerve, causes a readily identifiable, surprising pupillary light reaction (see Fig. 4-2). Swinging a flashlight from the normal eye to the one with optic neuritis will lead to dilation, rather than continued constriction, of both pupils. This paradoxical, unilateral reaction results from less light entering the pupillary reflex arc compared to when the light was shone into the normal eye. Sometimes called the “swinging flashing test,” this abnormality in the afferent limb of the light reflex results in an afferent pupillary defect or “Marcus Gunn” pupil, which neurologists interpret as a sign of optic nerve pathology.
Ocular Motility Abnormalities
MS also causes ocular motility abnormalities, including nystagmus and the characteristic internuclear ophthalmoplegia (INO), which is also known as the medial longitudinal fasciculus (MLF) syndrome. Either brainstem or cerebellar involvement can cause nystagmus. Although it is clinically indistinguishable from nystagmus induced by other conditions (see Chapter 12), MS-induced nystagmus typically occurs in combination with dysarthria and tremor (Charcot’s triad).
In MS-induced INO, MLF demyelination interrupts nerve impulse transmission from the pontine conjugate gaze centers to the oculomotor nuclei (Figs 15-3 and 15-4). The primary symptom of INO is diplopia on lateral gaze because of paresis of the adducting eye. INO is strong evidence of MS; however, systemic lupus erythematosus (SLE or lupus [see later]) and small basilar artery strokes may also cause it. In addition, Wernicke–Korsakoff syndrome, myasthenia gravis, and botulism can produce patterns of ocular muscle weakness that mimic INO. From a physiologic viewpoint, INO is analogous to a disconnection syndrome, such as conduction aphasia, in which communicating links are severed but each neurologic center remains intact (see Chapter 8).
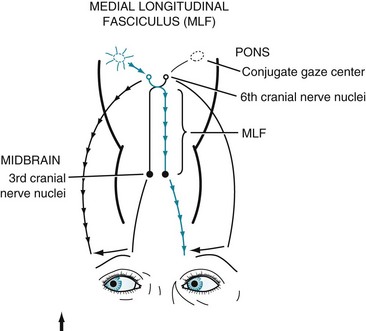
FIGURE 15-3 Under normal conditions, when looking laterally, the pontine conjugate gaze center stimulates the adjacent abducens (sixth) nerve nucleus and, through the medial longitudinal fasciculus (MLF), the contralateral oculomotor (third) nerve nucleus. For example, when looking to the right, as in this illustration, the right pontine gaze center stimulates the right abducens and the left oculomotor nuclei (see Fig. 12-12).
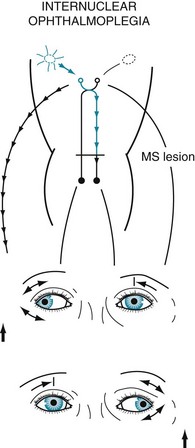
FIGURE 15-4 In internuclear ophthalmoplegia (INO), also known as the MLF syndrome, an interruption of the medial longitudinal fasciculus (MLF) prevents impulses from reaching the oculomotor (third) nuclei. Because those nuclei themselves remain intact, the pupils and eyelids are normal in both eyes. However, when looking to the right, because the left oculomotor nucleus is not stimulated, the left eye fails to adduct. The right eye abducts, but nystagmus develops. With bilateral INO, which is characteristic of multiple sclerosis (MS), neither eye adducts and abducting eyes have nystagmus.
Spinal Cord Symptoms and Signs
Spinal cord involvement also typically leads to urinary incontinence from a combination of spasticity, paresis, and incoordination (dyssynergia) of the bladder sphincter muscles (Fig. 15-5). MS patients initially often have incontinence during sleep and sexual intercourse. As the disease progresses, patients develop intermittent urinary retention and then complete loss of control. They often require intermittent or continuous catheterization, which leads to frequent, chronic, or recurrent urinary tract infections.
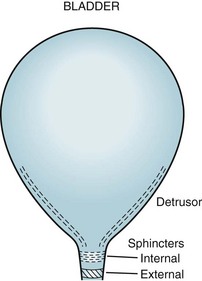
FIGURE 15-5 The urinary outflow of the bladder has two sphincters: an internal sphincter controlled by the autonomic nervous system (ANS), and an external one under voluntary control. Normal urinary bladder emptying (urination) occurs when the detrusor (wall) muscle contracts and both sphincter muscles relax. Purposefully urinating requires voluntary action (to relax the external sphincter) and reflex parasympathetic (ANS) activity (to contract the detrusor and relax the internal sphincter). Urinary retention occurs with either anticholinergic medication or excessive sympathetic activity because both inhibit detrusor contraction and internal sphincter muscle relaxation. Urinary retention also occurs with spinal cord injury because the external sphincter is unable to relax because it is spastic and paretic (dyssynergic).
Erectile dysfunction, decreased desire, and other forms of sexual impairment plague the majority of MS patients (see Chapter 16). About 40% of women with MS do not engage in sexual intercourse. Even before developing erectile dysfunction, men often experience premature or retrograde ejaculation. Sexual dysfunction, with or without urinary incontinence, is attributable to MS involving the spinal cord. With spinal cord damage severe enough to cause paraplegia, men have lowered and abnormal sperm production, but women can conceive and bear children.
Fatigue and Other Important Symptoms
MS-induced fatigue represents a physiologic cause of the chronic fatigue syndrome (see Chapter 6). Although many, but not all, studies found that depression is comorbid, antidepressants do not alleviate MS-induced fatigue.
Various pain syndromes also commonly occur in MS. For example, approximately 2% of MS patients suffer from trigeminal neuralgia (see Chapter 9) and 10% Lhermitte’s sign. Many MS patients have pain in their limbs or trunk. These pains probably arise from MS plaques irritating CNS pain-transmitting fibers in, respectively, the brainstem and cervical spinal cord. As with other forms of neuropathic pain (see Chapter 14), antiepileptic drugs, such as gabapentin and carbamazepine, provide some relief.
Because MS, in general, spares CNS structures that contain little or no myelin, symptoms that originate in gray-matter injury rarely complicate the illness. For example, MS patients seldom develop signs of focal cerebral cortical dysfunction, such as seizures or aphasia. Similarly, because the basal ganglia, like the cerebral cortex, are devoid of myelin, MS patients almost never develop involuntary movement disorders (see Chapter 18).
Psychiatric Comorbidity in MS
Depression
Consultants may also encounter “MS-induced euphoria” – an elevation of mood clearly inappropriate to these patients’ disability. This euphoria is associated with physical deterioration, chronicity of the illness, and at least subtle intellectual impairment, as well as steroid treatment. Some euphoric patients are masking depression or protecting themselves with denial. Others simply sense relief as an MS attack subsides. Whether or not psychologic factors seem to explain the euphoria, extensive cerebral involvement usually underlies it. In particular, pseudobulbar palsy may explain pathological laughter (see Chapter 4).
Cognitive Impairment
Almost all MS patients in the initial phase of their illness have normal cognitive capacity. They satisfactorily complete their day-to-day functions, routine mental status evaluation, and Mini-Mental State Examination (MMSE) (see Fig. 7-1). However, more demanding measures, such as the Wechsler Adult Intelligence Scale (WAIS), Selective Reminding Test, and Halstead Category Test, reveal at least clinically silent deficits in 45–65% of MS patients.
Laboratory Tests
Imaging Studies
MRI – certainly the most valuable test – can readily reveal demyelinated areas indicative of MS plaques. The revised McDonald criteria call for combinations of one gadolinium-enhanced lesion or nine T2-weighted hyperintense MRI lesions located in various regions of the brain, particularly in the periventricular area (Figs 15-6 and 20-25), or spinal cord (Fig. 15-7). Although not pathognomonic, these hyperintensities are detectable in more than 90% of MS patients.
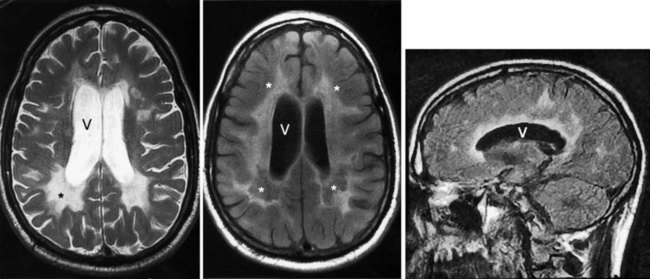
FIGURE 15-6 Left, This axial T2-weighted magnetic resonance imaging (MRI) scan through the cerebrum of a patient with multiple sclerosis (MS) shows multiple plaques (*) concentrated around the ventricles (V), particularly in the posterior regions. The MS plaques are characteristically white (hyperintense), sharply demarcated, and located in the periventricular region. Center, This axial T2-weighted, fluid-attenuated inversion recovery (FLAIR) image of the same study also shows hyperintense lesions (*) surrounding the ventricles (V). FLAIR images, in which cerebrospinal fluid remains black, highlight demyelinated areas. Right, The sagittal T2 FLAIR image of the same study shows the periventricular hyperintensities surrounding the lateral ventricle (V).
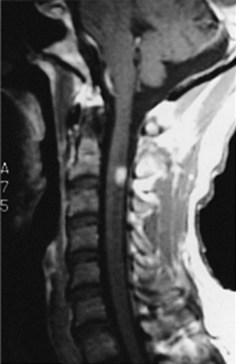
FIGURE 15-7 This magnetic resonance imaging scan of a patient with multiple sclerosis reveals a plaque – the hyperintense lesion – in the high cervical spinal cord.
Cerebrospinal Fluid
Routine CSF analysis during an MS attack will usually contain protein concentrations that are either normal (40 mg/100 mL) or only slightly elevated, and a mild, nonspecific gamma globulin elevation (9% or greater), but no increase in white blood cells (WBCs). One suggestive feature is that the CSF of 90% of MS patients contains CSF oligoclonal bands that consist of discrete IgG antibodies (Fig. 15-8). However, oligoclonal bands are also present in other inflammatory diseases involving the CNS, such as lupus, chronic meningitis, sarcoidosis, neurosyphilis, Lyme disease, acquired immunodeficiency syndrome (AIDS), and paraneoplastic limbic encephalitis.
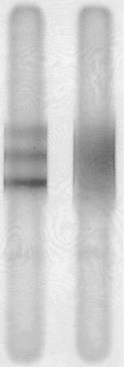
FIGURE 15-8 Electrophoresis of cerebrospinal fluid (CSF) of a patient with multiple sclerosis (left), compared to the CSF of one with no central nervous system inflammatory disease (right), shows three distinct, horizontal oligoclonal bands.
Evoked Responses
Visual-evoked responses (VERs) reveal visual pathway lesions. The patient stares at a rapidly flashing pattern on a television screen and a computer averages responses detected over the occipital cortex. Optic neuritis increases the latency or distorts the waveform. Because VERs can indicate the site of an interruption in the visual pathway, they are helpful in distinguishing ocular, cortical, and psychogenic blindness (see Chapter 12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








