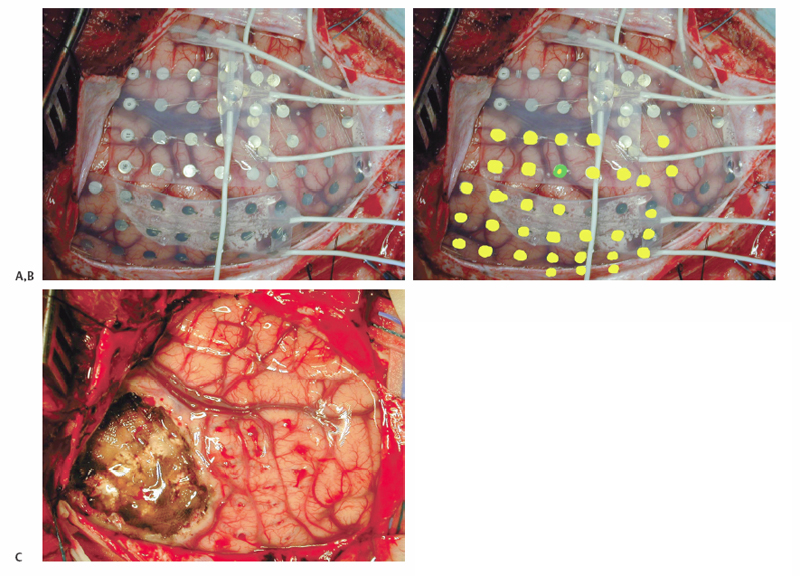
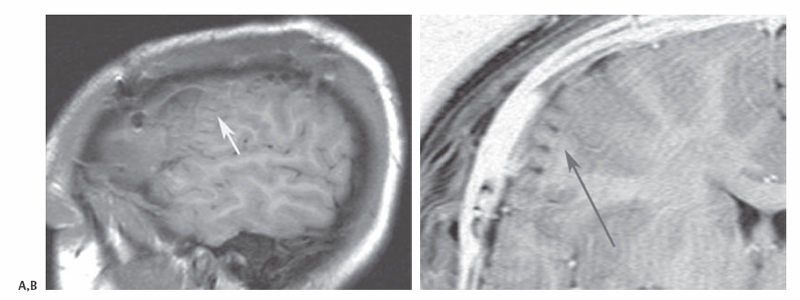
10 Multiple Subpial Transections The initiation and propagation of epileptiform discharges in the brain divides the problem of focal epilepsy into two parts: one, the generation of epileptiform activity, and two, the spread of epileptiform activity throughout the brain. Most successful epilepsy surgery is directed at resective techniques, that is, removing the seizure generator. Multiple subpial transection (MST) is a nonresective technique developed for the surgical interruption of the seizure process in areas of the brain where resective techniques would result in undesirable complications. Understanding the rationale behind MSTs is critical for the practitioner to perform them effectively. Morrell and Hambrey1 and Morrell et al2 conceived of the procedure of MSTs after an eruption in the understanding of multicellular neurophysiology. The concept of the cortical column developed in visual, auditory, somatosensory, and motor cortex physiology. Five millimeter–wide columns of neurons appeared to act as functional modules responsible for processing behavioral tasks. Vertically oriented neuronal processes such as apical and basal dendrites, as well as centrifugal and centripetal axonal process, appeared to be the necessary and sufficient building blocks for cortical function. Morrell cited experiments reported by Sperry and Miner3 and Sperry et al4 to support the rational behind MSTs. In Sperry’s experiments, subpial transections or the insertion of mica plates in cat visual cortex were used to surgically create isolated vertical cortical columns; visual function did not appear to be interrupted. Morrell hypothesized that if these columns were the minimal units for function, then transecting the brain to no less than these 5 mm units might be enough to prevent seizure initiation and propagation. Morrell proposed parceling neocortex into its minimal functional volumes to undermine the initiation of seizure activity by reducing the volumes of cortex to neuron numbers not sufficient to generate the synchronized activity to trigger a seizure. With this cortical disconnection, even if synchronization occurred in an isolated cortical cube, it would not be able to propagate horizontally, synchronizing other neurons. MSTs are applied, generally, to nonlesional cortical tissue after electrocorticography is used in an awake monitoring unit for seizure localization. During intraoperative electrode implantation, digital photography should be used to correlate surface anatomy with electrode position. Frameless stereotactic magnetic resonance imaging (MRI)-guidance is also important for colocalization, especially in lesional cases. After video monitoring, photographic maps are used to register epileptogenic areas as well as co-register information from awake motor and sensory mapping done in the epilepsy monitoring unit. These cortical maps are then used in the operating room at the time of surgery and refined with further motor and sensory mapping (Fig. 10.1). At the second surgery for removal of electrodes and performance of MSTs and resection, additional photographs are taken to demonstrate the actual area where the resection and transections were performed. Postoperative MRI is useful to demonstrate no postoperative complications, the removal of lesional cortex for lesional cases, and to demonstrate the depth and area of the MSTs (Fig. 10.2). The neurologist and neurosurgeon can use the intraoperative images in the forum of the preoperative epilepsy planning conference to discuss the possible risks, benefits, and options of the extent of resection and whether to substitute MSTs for surgical resection in areas that might be eloquent. Reports from different centers describe multiple variations on the technique of MSTs.2,5–8 Generally, a small hole is made in the pia for the insertion of the transector. A no. 11 blade can be used or a hypodermic needle of various gauges. We coagulate the surface of the pia, only briefly and at low amplitude, with an irrigating bipolar coagulator before inserting a 25-gauge needle to provide the point of entry for the transector. Different centers also report various points of entry for the transector. Morrell et al described the point of entry for the transector at the sulcal border of the gyrus, where they inserted the transector as deep into the sulcus as possible.2 Our preference is to make the point of entry in the middle of the gyrus. The transector is then inserted twice into the same point of entry and swept toward opposite sulci. We avoid the sulcal border as a point of entry because of its vascularity. The transector can be a microsurgical ball probe, stainless steel wire bent appropriately and held on a hemostat, or a commercially available product such as those developed for the procedure (e.g., AD-Tech Medical Instrument Corp., Racine WI). Transectors of various levels of sharpness and angle have been described. Our preference is to use a 5-mm blunt microsurgical probe bent at ~100 degrees. The length of the probe should be kept to 4 to 5 mm to avoid penetrating deeply into the white matter and disrupting cortical blood supply. We do not recommend a sharp probe so as not to pierce the pia on sweeping the transector backward or not to catch on sulcal vessels. We occasionally also use longertipped transectors with more obtuse angles to reach deeper along sulci when necessary. Transections should be performed every 5 mm along a sulcus perpendicular to the gyri. As much as possible, coagulation should be avoided in eloquent areas. Using thrombin-soaked Gelfoam (Pfizer Inc., New York, NY) over the entry point of the transection stops most bleeding. Fig. 10.1 Intraoperative digital photography is used to create cortical maps to record electrode positions, correlate sensory and motor mapping with electrode recordings and frameless stereotactic lesion localization, and communicate to the neurology team intraoperative findings. (A) Right frontotemporal-parietal craniotomy. Grid and strip electrodes are positioned to obtain maximal recording coverage. Photograph is oriented with temporal lobe superior and frontal lobe anterior. (B) Yellow dots indicate the epileptic areas recorded in the epilepsy monitoring unit. The green and yellow dot is the lesional area presumed to be cortical dysplasia from the MRI, localized using MR-guided frameless stereotaxy. (C) Postresection and MST. The resection cavity is seen on the left bordered to the right by the motor and sensory areas, treated with MSTs (note the punctuate hemorrhages frequently seen).
Scientific Rationale
Technique
Seizure Localization
Operative Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








