Fig. 11.1
MS, acute phase. T2-weighted image, (a, c) demonstrate focal hyperintensities in the left pons and in the white matter of the right hemisphere, respectively; note, edema around the plaque located in parietal region. After contrast medium administration, (b, d) enhancement is present in the active lesions
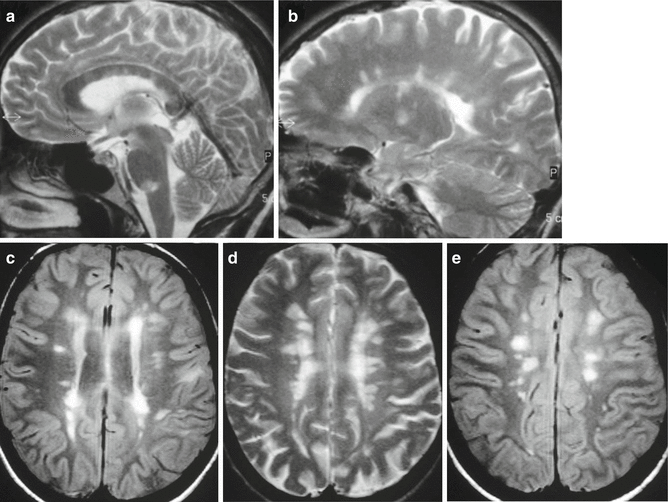
Fig. 11.2
MS, typical findings in different cases. Sagittal, (a, b) and axial, (c–e) T2-weighted images reveal typical numerous inflammatory demyelinating plaques located in the corpus callosum, in the brainstem, in the periventricular white matter and in perivenular spaces. Several lesions are confluent
11.3 Terminology
CIS – Acute or subacute first and single clinical event suggestive of an inflammatory demyelinating disease of the CNS. Typically, it involves a young adult and lasts at least 24 h, in absence of fever, infection or clinical aspects of diffuse encephalopathy.
The evolving definition of CIS related to the main question for the physician is whether the CIS is only an isolated episode or is the first episode of MS?
The definition of CIS has evolved in relation to progressive changes of criteria for MS diagnosis, mainly driven by the evolving relevance of MRI data to define the dissemination in space and time, and the characteristics that define the presence (or evolution) of a definite MS [1, 2].
Characterisations of CIS:
CIS ‘pure’, consists of one symptom and one corresponding MRI alteration; CIS isolated in space (monofocal) and in time (monophasic), without dissemination in space or time of an underlying disease
CIS with high probability of evolving into a clinically definite MS
Multifocal T2 abnormalities in the cerebral white matter (50–70 % of patients with CIS)
Multifocal onset
Inflammatory and immunological abnormalities in CSF
Radiological isolated syndrome (RIS): presence of brain MRI features suggestive of a demyelinating disease with or without non-specific symptoms. Thirty-40 % of cases will show characteristics typical of CIS or MS within 5 years. Brain lesions should fulfil MRI criteria for MS with regard to number of lesions (more than 9), location (periventricular, juxtacortical, infratentorial) and gadolinium enhancement.
Demographics – Sex ratio: female/male: 2.5/1. Mean age at onset 30 years (20–40)
11.4 Clinical Features
A clinical episode consistent with damage to white-matter tracts in young patients
Data from large database: 21 % caused optic neuritis, 46 % long-tract symptoms and signs, 10 % brainstem syndromes and 23 % multifocal abnormalities
11.5 Diagnosis
11.5.1 Imaging
One or more hyperintense lesions at T2-MRI
Possible lesion enhancement in T1 with gadolinium
Brain and spinal cord imaging
Increased optic nerve volume in optic neuritis, with subsequent nerve atrophy (Fig. 11.3)
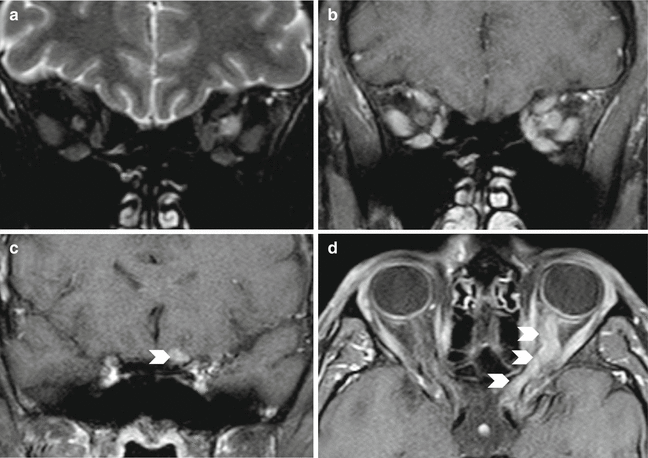
Fig. 11.3
NORB. Acute phase. Coronal T2-weighted image (a) shows enlargement and hyperintensity of the left optic nerve, and after contrast medium coronal image shows intense enhancement. (b) Enhancement of the intraorbital and intracranial left optic nerve is well documented in coronal and axial T1-weighted images after contrast medium (c, d) (arrowheads)
11.5.2 Laboratory
CSF – oligoclonal bands in two-thirds of CIS
Lack of a validated immunological marker to predict the development of MS
11.5.3 Top Differential Diagnosis
1.
CIS in inflammatory demyelinating disease and MS.
2.
MS: following the 2010 revision of McDonald criteria [1], MS can be diagnosed at the time of CIS, if other silent demyelinating lesion are found.
CIS overlapping other diseases (neuromyelitis optica, neurosarcoidosis, LES vasculitis).
11.6 Principle of Treatment
11.6.1 Treatment of CIS [3]
Many patients with CIS recover spontaneously.
Intravenous steroids are often employed in cases with persistent symptoms (usually 3–5 day course of intravenous methylprednisolone with or without subsequent oral prednisone for 9–12 days). Steroids increase the speed and the amount of improvement in optic neuritis, the most studied variety of CIS.
The efficacy of plasma exchange or intravenous immunoglobulin is controversial in CIS treatment.
Immunomodulatory treatment for the prevention of MS:
Randomized, double-blind and placebo-controlled trials of interferon beta and glatiramer acetate in patients with CIS at risk of MS showed a delay in the development of clinically definite MS in the treated patients.
Immunomodulatory treatment is usually started a second clinical episode in most countries, but it could be prescribed in CIS at risk of MS.
11.7 Prognosis
11.7.1 Evolution to Definite MS [4]
Presence of clinical or radiological dissemination in space and time.
Probability of 17–45 % of a second episode in 2 years, about 50 % at 5 years, 60 % at 10 years and 68 % at 14 years. The median time to second episode is about 2 years.
11.7.2 Prognostic Factors for Evolution to MS
Multiple lesions at first MRI evaluation (present in 50–70 % of CIS) with 50 % of conversion rate for optic neuritis and 90 % for other CIS in prolonged follow-up. In the cases of a unique lesion, the long-term risk of conversion to clinically definite MS is about 20 %. Both localization and number of lesions could be relevant for the risk of conversion, with infratentorial lesion at onset responsible for an increased risk.
Younger age.
Signs of inflammation in the CSF.
Raised cell count.
Oligoclonal bands (two or more oligoclonal bands in the CSF without corresponding bands in the serum).
Multiple functional systems affected at onset.
Other prognostic factors:
Cortical lesions at MRI
Atrophy of grey and white brain matter
11.7.3 Prognostic Factors for Long-Term Disability
Spinal onset
Polysymptomatic onset
Efferent long pathways involvement at presentation
Multiple lesions at first MRI evaluation
Brain atrophy
Incomplete remission of the clinical impairment after the first episode
11.8 Relapsing Remitting MS (RRMS)
11.8.1 Definition and Demographics
Relapsing Remitting (RRMS) is the most common MS phenotype involving around 85 % of patients. Clinical symptoms typically occur between 20 and 40 years of age. The frequency of MS has increased over the past century, mostly in women.
11.8.2 Clinical Features
Episodes of neurological impairment followed by complete or nearly complete recovery are typical of RRMS. In accordance with the different CNS regions involved, patients characteristically develop multiple functional problems such as visual and sensory disturbances, limb weakness, walking abnormalities, bladder and bowel symptoms and cognitive impairment.
Clinical stages: RR-active is determined by clinical relapses and/or MRI activity (contrast-enhancing lesions; new or unequivocally enlarging T2 lesions assessed at least annually). Periods of clinical stability between relapses define RR-not active [5]. In clinical trials different levels of severity have been used:
Active RRMS: defined by two clinical relapses in the previous 2 years. It is the most common clinical stage.
Highly active RRMS: defined by at least one relapse in the previous year while on therapy, and at least nine T2-hyperintensive lesions on brain magnetic resonance imaging (MRI) or at least one gadolinium-enhancing lesion.
Rapidly evolving severe RRMS: defined by two or more disabling relapses in 1 year, and one or more gadolinium-enhancing lesions on brain MRI or a significant increase in T2 lesion load compared with a previous MRI.
11.8.3 Diagnosis
MS is diagnosed according to the revised McDonald’s criteria, which incorporate results of MRI [1]. The new diagnostic criteria include:
Evidence of damage in at least two separate areas of the CNS
The damage occurs at least 1 month apart
Exclusion of other possible diagnoses
As a consequence, the diagnosis can be made earlier, at the first manifestation suggestive of MS (CIS), since the dissemination in space or time can be replaced by MRI findings even if clinical manifestations are absent. This has drastically changed the MS population included in clinical trials and clinical therapeutic decisions.
If the McDonald’s criteria are fulfilled and there is no better explanation for the clinical syndrome, the diagnosis is ‘MS’; if suspicious, but the criteria are not completely met, the diagnosis is ‘possible MS’; if another diagnosis arises during the evaluation that better explains the clinical presentation, then the diagnosis is ‘not MS’ [1].
Brain MRI detects dissemination of lesions both in space and time. At least three of four of the following MRI diagnostic criteria are required for the diagnosis of MS:
One gadolinium-enhancing lesion or nine T2-hyperintense lesions in the absence of gadolinium-enhancement
At least one infratentorial lesion
At least one juxtacortical lesion
At least three periventricular lesions
The ascertainment of dissemination in time by MRI requires a new T2 and/or gadolinium-enhancing lesion(s) at follow-up MRI, with reference to a baseline scan, irrespective of the timing of the baseline MRI, or simultaneous presence of asymptomatic gadolinium-enhancing and non-enhancing lesions at any time. Spinal cord MRI may be useful if brain MRI does not identify dissemination in space in a patient suspected of having MS.
CSF – oligoclonal IgG bands different from those found in serum and/or elevated immunoglobulin G index (not specific of MS and not essential for its diagnosis). Mild pleocytosis. The additional value of CSF for MS diagnosis is still under discussion [1].
Neurophysiology – Multimodal and, particularly, visual evoked potentials (VEP) may be abnormal.
11.8.4 Pathology
The main pathological events in the CNS include focal lymphocytic infiltration and microglia activation followed by demyelination and axonal degeneration.
11.8.5 Top Differential Diagnoses
Neuromyelitis optica (NMO) Acute disseminated encephalomyelitis (ADEM) (see Chapter …Marchioni).
11.8.6 Therapy
Relapses are treated with intravenous methylprednisolone 1 g daily for 3–5 days.
Disease modifying drugs (DMDs): 11 DMDs are licensed for RRMS. Six of them are first-generation drugs (employed over the last 20 years): interferon beta-1a (Avonex and Rebif), interferon beta-1b, glatiramer acetate natalizumab and mitoxantrone (since 2000). Five new DMDs have been licensed over the last 5 years: fingolimod (since 2011), teriflunomide and alemtuzumab (since 2013), dimethyl fumarate and pegylated interferon beta-1a (since 2014).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





