22 Natural History of Giant Intracranial Aneurysms
Introduction
The first clinical series of giant intracranial aneurysms were reported in 1969 by Morley and Barr1 and Bull.2 Giant intracranial aneurysms (GIAs) are defined as those aneurysms with a greatest diameter equal to or exceeding 2.5 cm. This arbitrary designation was chosen in order to conform to the subset of the largest aneurysms in the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage.3 Likewise smaller lesions have been shown to differ significantly with regard to their rate of rupture, the incidence of presentation with mass effect, and most importantly the difficulty with surgical treatment. GIAs are relatively infrequent compared to their smaller counterparts and therefore literature support to aid in clinical decision making is not as readily available. While once thought to represent more benign lesions with a lower incidence of hemorrhage and a more optimistic natural history, it is clear now from recent, large, multicenter studies that these lesions behave aggressively and hemorrhage more frequently than their smaller counterparts.4,5
Pathologic considerations
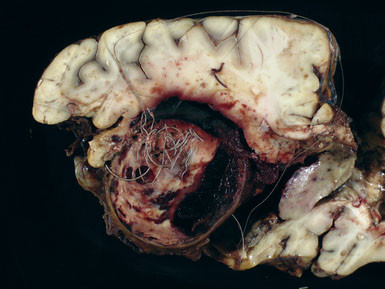
Giant aneurysms are classified as either saccular or fusiform with the vast majority being the saccular type. Giant saccular aneurysms develop as a result of hemodynamic stress at arterial bifurcations and branch points similar to smaller saccular intracranial aneurysms. Once the sac has formed, both the neck and fundus will then undergo progressive enlargement. Ferguson proposed that turbulence within the aneurysm leads to endothelial injury and subsequent platelet aggregation and fibrin deposition.6 This intraluminal cascade in giant aneurysms represents a dynamic series of events with accumulation and dissipation of platelets and fibrin-thrombus debris in an irregular fashion. Fusiform giant aneurysms most commonly result from atherosclerosis, however, may develop in patients with collagen vascular disorders such as Marfan’s syndrome, Ehlers-Danlos syndrome, and systemic lupus erythematosis. All of these diseases produce multifocal injury to the endothelial wall, resulting in weakening of an entire segment of the vessel. Fusiform lesions often present because of mass effect but not infrequently produce ischemic symptoms secondary to thromboembolic phenomena (Figure 22–1).
Epidemiology
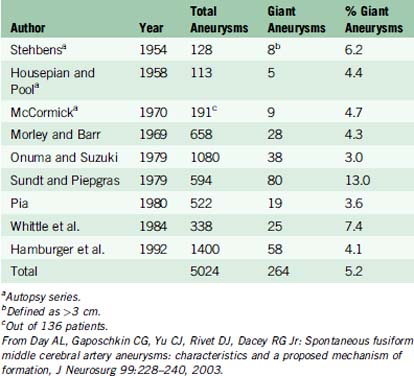
Some epidemiologic differences from smaller saccular aneurysms seem to validate the separate clinical entity of giant aneurysms. In various clinical and autopsy series, the percentage of total aneurysms that are giant ranges from 3% to 13%. In one of the largest series to date, Hamburger et al. reported 1400 intracranial aneurysms and identified 58 giant aneurysms, yielding an incidence of 4.1%.11 Based on data from three autopsy studies and six large clinical series, the aggregate incidence of giant intracranial aneurysms is 5.2%13 (Table 22–1).
Age and gender
GIAs more commonly occur in females and most commonly come to clinical attention during the fifth to sixth decade of life. In a review of the world literature, Fox identified 693 giant aneurysms of which 60% occurred in females. This review correlates with known anatomical gender distributions with the proximal internal carotid artery location in 73% of females versus 52% for the anterior communicating complex and basilar apex.7 Anson reviewed 14 series with 754 GIAs identified, finding 461 females and 295 males, yielding a 1.56:1 ratio.27 In Anson’s review, ages ranged from 6 months to 76 years with 67% of patients presenting between ages 40 and 70. This distribution parallels that of Fox’s review of the world literature, where the median age of presentation was in the sixth decade of life.
Giant fusiform and dolichoectatic aneurysms probably present at a slightly younger age. Reviews from three different institutions found a mean age of presentation to be 38, 43, and 49 years with a male:female ratio of 1.5:1.8–10
Intracranial aneurysms are rare in children, with only 1% to 2% of all aneurysms occurring in the pediatric population. Of these, however, GIAs constitute a much higher proportion, are more common in males, and are less often present with hemorrhage. Ferrante reviewed 72 cases of pediatric aneurysms and found that 27% were giant and 50% were 1 to 2.5 cm in diameter.11
Location
Saccular GIAs have a similar anatomic predilection as their smaller counterparts, while the distribution is different. Similarly, the internal carotid artery (ICA) is the most common site, although GIAs occur proportionately more frequently in the vertebrobasilar system and less frequently in the anterior communicating artery (ACOM) region. Drake and Peerless reported 641 GIAs treated between 1965 and 1992 in the following locations: vertebrobasilar, 390; ICA, 178; middle cerebral artery (MCA), 64; and anterior cerebral artery (ACA), 19.12 While their data does reflect a referral bias, it supports the general trend that certain intracranial arterial segments are more vulnerable to this pathology as seen throughout the literature. Giant fusiform and dolichoectatic aneurysms have a predilection for the vertebrobasilar circulation and the MCA. These lesions, frequently referred to as “serpentine aneurysms,” are often massive, partially thrombosed lesions through which runs a serpentine channel.13
Natural history
The natural history of GIAs is dependent upon several factors, including the location of the aneurysm with respect to the subarachnoid space, the pathological form of the aneurysm (saccular vs. fusiform), the specific anatomic location, and the presence or absence of laminated thrombus and or atherosclerotic plaque within the fundus and neck of the aneurysm.14 In Morley and Barr’s original series of 28 patients, 17 had intradural aneurysms, and only four of those underwent a direct operation on their aneurysm. Of the five patients who received no definitive therapy, four died. Of the five patients with intradural aneurysms who had common carotid ligation, three died and one was disabled. They concluded that “direct surgical attack on extracavernous giant aneurysms is seldom possible or successful except in the case of MCA aneurysms.”
Peerless et al. observed 31 patients with giant intracranial aneurysms (25 saccular and six fusiform). Sixty-eight percent of patients with saccular aneurysms were dead at 2 years and 85% were dead at 5 years. If patients presenting with subarachnoid hemorrhage were excluded, the 2-year mortality rate was 62%. Only four patients who were all disabled were alive at 5 years. Of the six patients with fusiform aneurysms, four were dead at 2 years, one died at 3.5 years after diagnosis, and one remained disabled.15 Ljunggren et al. demonstrated a mortality or severe morbidity rate of 80% within 5 years in patients with untreated symptomatic GIAs.16 Hamburger et al. reported on 58 patients with GIA and showed that 7% of patients presenting without SAH died, compared with 29% of patients presenting with SAH. Only 18% of the later group were discharged home as independent compared with 50% of the former group.17 The Italian Cooperative Study on Giant Intracranial Aneurysms found that extensive or thick cisternal deposition of blood was associated with a significantly higher mortality rate than with thin or absent depositions.18 Although GIAs present more commonly with mass effect or thromboembolic events, there is no evidence that they are associated with a lower rate of rupture. It is evident that the presence of thrombus in the sac does not protect against SAH and that when extensive or complete may actually have a deleterious effect on the natural history.19–21
The most recent and comprehensive data on the natural history of GIAs comes from the International Study of Unruptured Intracranial Aneurysms Investigators (ISUIA I & II).4,5 In the retrospective arm, ISUIA I, 1449 patients with 1937 intracranial aneurysms were reviewed. The patients were divided into two groups. Group 1 patients (N = 727) had no history of SAH from a different aneurysm, while Group 2 (N = 722) had a history of a SAH from a previously repaired aneurysm. The rupture rate for aneurysms greater than 25 mm in diameter was 6% in the first year. In the prospective arm, ISUIA II, 4060 patients were assessed of which 1692 did not have aneurysmal repair. The 5-year cumulative rupture rates for patients who did not have a history of SAH was 40% for giant aneurysms located in the anterior circulation, and 50% for aneurysms located in the posterior circulation as well as the posterior communicating artery (PCOM).
The natural history of patients presenting with symptoms related to mass effect or ischemia from giant aneurysms is less clear. In giant paraclinoid aneurysms presenting with visual loss, the deterioration usually progresses without treatment.22,23 Patients with symptoms of brainstem compression due to giant dolichoectatic verterbobasilar artery aneurysms also appear to have a poor prognosis without treatment. In a report by Michael, all seven patients with giant posterior circulation aneurysms treated with observation died between 2 months and 2 years after diagnosis.24 The Italian Cooperative Study analyzed 25 patients with untreated giant aneurysms and reported mortality in seven cases (28%), which was double the mortality rate in the 86 treated cases. Morbidity was seen in 12 of the observed cases due to progressive expansion of the aneurysm mass.
The natural history of giant aneurysms presenting with mass effect is usually one of progressive enlargement. Sonntag and Stein demonstrated progressive enlargement on successive angiograms in two of 13 cases being non-surgically managed.25 Growth can be extremely rapid with one case in the literature documenting formation in less than 3 months.26 There is no evidence that the presence of extensive thrombus improves the natural history of giant aneurysms. Despite the lack of luminal filling, it has been demonstrated that even extensive intra-aneursmal thrombosis does not protect against SAH. Furthermore, the presence of extensive or even complete thrombosis within a giant aneurysm may increase the risk of compressive symptoms.27
The risk of distal ischemic symptoms may actually increase in partially or extensively thrombosed giant aneurysms, contributing to a more ominous natural history. Sutherland and Peerless noted distal thromboembolism in 59% of cases in their series of giant aneurysms containing thrombus.28 In addition, it is also possible for thrombus to propagate into the parent artery and cause occlusion. The risk of thromboembolic stroke from thrombosed giant aneurysms seems to be greatest in cases of fusiform or dolichoectatic vertebrobasilar artery aneurysms.29