Natural Products with Anti-Addictive Activities
David Yue-Wei Lee
There are two major therapeutic trends in the United States. The first involves the use of a sophisticated molecular approach for the development of novel medications that specifically target certain disease genes, receptors, or enzymes. The second involves the use of holistic approaches with herbal medicine. The latter trend has gained increasing popularity because Western medicine has been ineffective in certain chronic diseases, may produce adverse effects, or may be too costly. Interestingly, both Western medicine and herbal medicine share the common belief that human health is associated with functional balance. Western medicine provides fast relief of symptoms at the disease site, particularly under critical conditions, but the single chemical entity usually targeting a single receptor site may not be sufficient to restore the functional balance of the entire body. On the other hand, herbal medicine, with a multitargeted approach, focuses on the functional balance of the body in a holistic manner. Therefore, in a broader perspective, herbal medicine may have advantages in dealing with chronic diseases. Drug addiction, including involuntary addiction to prescription pain killers, is one of the most challenging problems in medicine. It is considered a mental disease that requires long-term treatment with psychopharmacotherapies such as methadone replacement therapy, which is still far from ideal. Chinese herbal medicines have been adopted in the treatment of opium addiction in China since 1850 and the effectiveness of such traditional treatments in several Asian countries was reported (1, 2, 3). It is possible that certain Chinese herbal remedies developed during the opium war era may provide a rich source for the discovery of natural products with the potential for the treatment of drug abuse in the 21st century. This chapter focuses on natural products with anti-addictive activities.
BACKGROUND
The poppy has been known in China for 12 centuries and has been used for medicinal purpose for nine; however, it was not until the middle of the 19th century that smoking opium for recreational purposes was practiced throughout China. The quantity of opium imported into China was on the order of 5,000 chests in 1820. This amount steadily increased to 16,000 chests in 1830; 20,000 chests in 1838; and 70,000 chests in 1858. After more than 100 years of social decline, and with half of the population addicted to opium—an amount equal to almost the entire U.S. population today—Chinese authorities finally determined to force the people to give up opium, which ultimately led to the Opium Wars. During this period, Chinese herbal remedies were developed for the purpose of treatment of opium addiction and relieving withdrawal syndromes induced by stopping opium use.
Opiates, a novel class of alkaloids derived from poppy, can effectively activate the endogenous opiate system in the body. This activation produces many cardiovascular, endocrine,
immune, and neuropsychologic effects including euphoria, analgesia, and addiction. Heroin is a well-known example of this group of compounds. It is clear that the effects of opiate drugs are mediated through interaction with opioid receptors. Moreover, studies on the binding of various related opiate compounds in the brain indicate the existence of a multitude of opioid receptor types and subtypes such as mu, kappa, and delta (4). Since the rewarding effects of opiates are mediated through action at mu-opioid receptors, interference with actions at these receptors presents a rational strategy for developing medications for opiate addiction (5,6). Specifically, medications that block activation of mu-opioid receptors (e.g., naltrexone) and kappa receptors might reduce drug-seeking behavior (4,6,7). Since the Chinese remedies developed during the opium war era were combinations of more than a dozen herbs, it is conceivable that such a mixture of herbs may well represent a multitargeted approach acting on opioid receptors, which would have the benefits of improved overall efficacy with reduced toxicity. However, it is necessary to engage in the isolation and characterization of the bioactive compounds, and to elucidate the mechanism of actions for further development of a safe and complementary natural medication for drug abuse.
immune, and neuropsychologic effects including euphoria, analgesia, and addiction. Heroin is a well-known example of this group of compounds. It is clear that the effects of opiate drugs are mediated through interaction with opioid receptors. Moreover, studies on the binding of various related opiate compounds in the brain indicate the existence of a multitude of opioid receptor types and subtypes such as mu, kappa, and delta (4). Since the rewarding effects of opiates are mediated through action at mu-opioid receptors, interference with actions at these receptors presents a rational strategy for developing medications for opiate addiction (5,6). Specifically, medications that block activation of mu-opioid receptors (e.g., naltrexone) and kappa receptors might reduce drug-seeking behavior (4,6,7). Since the Chinese remedies developed during the opium war era were combinations of more than a dozen herbs, it is conceivable that such a mixture of herbs may well represent a multitargeted approach acting on opioid receptors, which would have the benefits of improved overall efficacy with reduced toxicity. However, it is necessary to engage in the isolation and characterization of the bioactive compounds, and to elucidate the mechanism of actions for further development of a safe and complementary natural medication for drug abuse.
Medicinal plants have been used for the treatment of human disease for over 2,000 years. It is estimated that roughly one-half of current pharmaceuticals originally were procured from plants (8). Examples include foxglove leaf (digitalis), belladonna tops (atropine), poppy herb (morphine), white willow tree bark (salicin), and cinchona bark (quinine). Modern drugs developed from plant products include warfarin from coumarine anticoagulants found in sweet clover silage, ergotamine from the ergot alkaloids of a fungus that infects rye grass, antineoplastic vincristine from the vinca alkaloid fractions of the rosy periwinkle, the anticancer drug taxol from pacific yew tree, and antimalaria artimisine from qingao (9). In contrast to such individual natural products isolated from plants, many believe that a combination of medicinal plants with synergistic effects of the various components of each plant may work the best. Although it is difficult to prove the synergism of multicomponent herbal remedies, the concept of a multitargeted approach has been evidenced by a number of studies using modern pharmacologic receptors. It is also conceivable that a multitargeted approach would improve overall efficacy with reduced toxicity. For example, Chung et al. (10) reported the in vitro receptor-binding affinities of natural products used successfully to treat psychotic illness in Korean traditional medicine.
Based on pharmacologic and anatomic analyses, multiple types of opioid receptors (at least μ, δ, and K) were elucidated in the late 1970s and 1980s (11,12), as well as opioid drugs and endogenous peptides acting on opioid receptors to produce pharmacologic and physiologic effects. Stimulation of opioid receptors, acting mostly via pertussis toxin-sensitive G proteins, affects a variety of effectors, which include adenylyl cyclase, potassium and calcium channels, phospholipase C, and p42/p44 mitogen-activated protein (MAP) kinase pathway (13). Following the cloning of the mouse k-opioid receptor (14,15), μ, δ, and K receptors of several species were cloned (16,17). These cloned opioid receptors provide excellent tools for pharmacologic screening of these traditional remedies for drug abuse. For instance, the extract YGT (NPI-025), prepared from five Chinese medicinal plants (Qiang Huo, Gou Teng, Chun Xiong, Fu Zi, and Yan Hu Suo) and used clinically in Hong Kong (18,19), was subjected to bioactivity guided fractionation and showed potent opioid receptor binding activities to μ, k, D1, and D2. These studies provide important scientific evidence for further development of such natural products.
MEDICINAL PLANTS WITH ANTI-ADDICTIVE ACTIVITIES
Chinese herbal remedies developed for the purpose of treatment or relieving withdrawal syndromes induced by opium often contain a complex mixture of Chinese medicinal plants.
Unfortunately, these herbal remedies have not been investigated with modern pharmacologic in vitro and in vivo models. Thus far, only the formula of YGT (NPI-025), studied in Hong Kong by Dr. Yang, both in animals and in humans, has obtained encouraging results. NPI-025 was used to treat 300 drug addicts in Hong Kong over a 10-year period. The clinical results of 100 cases for which there were comparatively complete records were reported by Yang et al. (19) in 1985. NPI-025 significantly reduced the withdrawal symptoms (—48%). The follow-up visits of many “cured” patients 1 to 3 years after treatment revealed that NPI-025 was helpful in controlling their craving for drugs. A number of selected medicinal plants used in China for substance abuse treatment are discussed below.
Unfortunately, these herbal remedies have not been investigated with modern pharmacologic in vitro and in vivo models. Thus far, only the formula of YGT (NPI-025), studied in Hong Kong by Dr. Yang, both in animals and in humans, has obtained encouraging results. NPI-025 was used to treat 300 drug addicts in Hong Kong over a 10-year period. The clinical results of 100 cases for which there were comparatively complete records were reported by Yang et al. (19) in 1985. NPI-025 significantly reduced the withdrawal symptoms (—48%). The follow-up visits of many “cured” patients 1 to 3 years after treatment revealed that NPI-025 was helpful in controlling their craving for drugs. A number of selected medicinal plants used in China for substance abuse treatment are discussed below.
Pueraria lobota (kudzu)
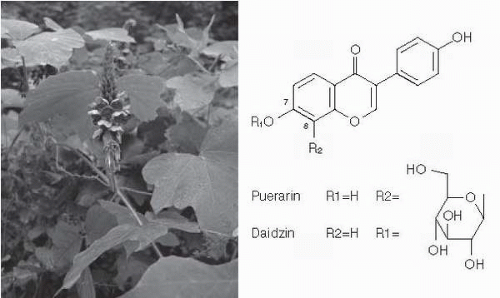
Pueraria lobota (kudzu) has traditionally been used in China for the treatment of alcoholinduced toxicity and stroke (20). In a recent study, daidzin and puerarin (Fig. 13.1), isolated from kudzu and given orally, suppressed voluntary alcohol consumption by alcohol-preferring P rats (21). Puerarin is the most highly concentrated isoflavone in P. lobata and because the sugar moiety is attached through a carbon-carbon bond, which is much more resistant to metabolism than daidzin, another isoflavone in P. lobata, it has also been shown to reduce alcohol intake in alcohol-preferring rats and hamsters (22,23). These findings, together with the reports of the effective reduction of drinking by kudzu extract (NPI-028) (24, 25, 26), provide a strong scientific basis for the traditional use of kudzu in the treatment of alcohol intoxication in China. However, the mechanisms of action of these isoflavones have not yet been examined. Recently, the potential anxiolytic effect of these isoflavones has been demonstrated (27, 28, 29). It is conceivable that puerarin may be a useful adjunct in detoxifying alcoholics, particularly if benzodiazepines can be avoided.
PHARMACOLOGIC ACTIONS
Effects on Ethanol Withdrawal in Rats
A modified pair-feeding design was used in this investigation. Rats maintained on a control diet (CD) were given a volume of food equivalent to the average volume consumed the previous day
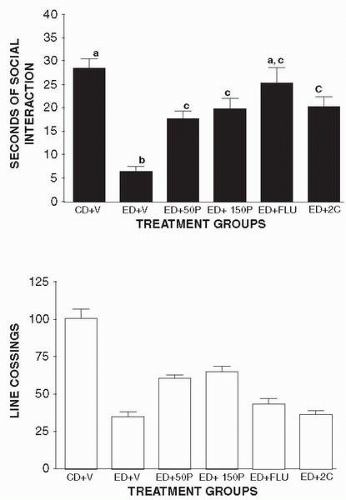
by rats maintained on an ethanol diet (ED). The rats were weighed at weekly intervals and volumes of diet were adjusted to ensure that the groups had similar body weights. Behavioral assessments were conducted after 17 days of exposure to the ED between 5 and 6 hours after the removal of the ethanol. This time point was selected on the basis of previous observations of anxiety-like behavior (30, 31, 32). Approximately 4.5 hours later rats received injections of one of the following treatments: CMC vehicle (8 CD controls and 8 ED rats), puerarin (8 rats at 50 mg per kg and 8 rats at150 mg per kg); and SB 242084 (8 rats at 1 mg per kg). Flumazenil (8 rats at 5 mg per kg) is very short-acting, so it was injected just 7 minutes before the behavioral test. Pairs of rats were placed into the social interaction arena for a 5-minute session 5 hours after ethanol was withdrawn. The resulting effects on social interaction are discussed on the next page.
by rats maintained on an ethanol diet (ED). The rats were weighed at weekly intervals and volumes of diet were adjusted to ensure that the groups had similar body weights. Behavioral assessments were conducted after 17 days of exposure to the ED between 5 and 6 hours after the removal of the ethanol. This time point was selected on the basis of previous observations of anxiety-like behavior (30, 31, 32). Approximately 4.5 hours later rats received injections of one of the following treatments: CMC vehicle (8 CD controls and 8 ED rats), puerarin (8 rats at 50 mg per kg and 8 rats at150 mg per kg); and SB 242084 (8 rats at 1 mg per kg). Flumazenil (8 rats at 5 mg per kg) is very short-acting, so it was injected just 7 minutes before the behavioral test. Pairs of rats were placed into the social interaction arena for a 5-minute session 5 hours after ethanol was withdrawn. The resulting effects on social interaction are discussed on the next page.
Counteraction of Anxiogenic Effects of DMCM and Ro 60 0175
Male Sprague-Dawley rats were adapted to the laboratory conditions for 1 week and then assigned one of the following treatment groups: VV (vehicle followed by another vehicle 30 minutes later); VD (vehicle followed by 0.5 mg per kg DMCM, a benzodiazepine inverse agonist, 30 minutes later); PD (150 mg per kg puerarin [NPI-031G] followed by 0.5 mg per kg DMCM 30 minutes later); VR (vehicle followed by 0.3 mg per kg Ro 60 0175); PR (150 mg per kg puerarin [NPI-031G] followed by 0.3 mg per kg Ro 60 0175, a 5-HT2C agonist). Pairs of rats were injected in parallel and placed in the social interaction arena 30 minutes after the second injection. There were eight rats (four pairs) in each group. The resulting effects on social interaction are discussed below.
Effects on Social Interaction in Rats
The social interaction test was used in these studies because it can detect the anxiolytic and anxiogenic effects of serotonergic agents (33, 34, 35). A modification of the standard social interaction test was used to conserve animals. According to File (36), the most sensitive procedure is to match up pairs of rats that had the same treatment on the basis of their body weights and then treat the total number of interactions by the pair as the unit of measure. However, for other experiments where the index rat may have an implanted cannula (34,37), an untreated dummy partner is used and only the interactions of the index rat are recorded. In the current studies, pairs of rats with the same treatment were placed in the arena and the social interactions initiated by each member of the pair were recorded, thereby requiring fewer rats (29). Statistical analyses of several data sets revealed that this approach provided the same statistical outcome as treating the scores of the pair as a unit. Furthermore, in a study of 25 pairs of rats maintained on a CD and 25 on an ED, the rats exhibited essentially independent behavior, as there was no significant within-pair correlation in either group (0.03 for CD, −0.13 for ED). During the 5-minute session, line crosses (by two forepaws) and time spent in social interaction (grooming, sniffing, following, crawling over or under) were scored individually for each rat (29,38).
As shown in Figure 13.2, puerarin partially counteracted the reduced time spent in social interaction at two separate doses, as did flumazenil and SB 242084 (Fig. 13.2, upper panel). There were highly significant differences among the groups (F5,54 = 13.82, P < 0.0001), with the ethanol-withdrawn rats spending very little time in social interaction. Interestingly, puerarin also partially counteracted the reduction in line crossings induced by ethanol withdrawal, while flumazenil or SB 242084 did not (Fig. 13.2, lower panel). The one-way ANOVA indicated significant differences among the groups (F5,54 = 16.87, P < 0.0001) with the ethanol-withdrawn rats exhibiting the fewest line crossings. Thus, puerarin partially counteracts two of the major behavioral symptoms of ethanol withdrawal.
Puerarin may have counteracted the reduced social interaction exhibited by ethanol-withdrawn rats by blocking either benzodiazepine or 5-HT2C receptors or both. As illustrated
in Figure 13.3, puerarin partially counteracts the reduced social interaction behavior generated by either DMCM, the benzodiazepine inverse agonist, or Ro 60 0175, a 5-HT2C agonist. In a separate group of rats it was established that puerarin itself did not alter time spent in social interaction (23.8 ± 3.2 seconds), so its partial counteraction of the anxiogenic effects of DMCM and Ro 60 0175 cannot be explained by nonspecific additive effects. The most likely mechanism underlying the effects of puerarin is, therefore, as a weak antagonist at the BZD receptor.
in Figure 13.3, puerarin partially counteracts the reduced social interaction behavior generated by either DMCM, the benzodiazepine inverse agonist, or Ro 60 0175, a 5-HT2C agonist. In a separate group of rats it was established that puerarin itself did not alter time spent in social interaction (23.8 ± 3.2 seconds), so its partial counteraction of the anxiogenic effects of DMCM and Ro 60 0175 cannot be explained by nonspecific additive effects. The most likely mechanism underlying the effects of puerarin is, therefore, as a weak antagonist at the BZD receptor.
Salvia divinorum
There is an impressive array of plants whose leaves, roots, and flowers offer the psychedelic (mind-manifesting) potential of a visionary reality to those who are familiar with their folkloric mysteries. In the Western world, nearly 100 plants have been used over the years by various cultural subgroups in search of psychoactive phenomena. These plants are classified as hallucinogens and have similar characteristics for altering perception in a bizarre manner and dramatically influencing the state of mind (39). The ingenuity of human beings in discovering natural substances that alter consciousness seems never-ending. Mind-altering plants have
been found in virtually all parts of the world. Historically, these hallucinogens are predominately used for producing a mystical experience or a sense of communion with Gods. They were also believed to have special healing powers and to enhance sociability. LaBarre (40) reported that throughout recorded history, hallucinogenic plants were exalted to a sacred role in magico-religious rites. For instance, Aztecs achieved inspiration by eating peyote, called teonanacatl or Gods’ flesh. Similarly, shamans or Indian medicine men of the Rio Grande valley and central Mexico used peyote buttons in their tribal rituals. The earliest report of peyote suggests that the Chichimecas and Toltecs were acquainted with it as early as 300 B.C. (41). Peyote intoxication is characterized by brilliant colored visions in kaleidoscopic movement, often accompanied by auditory, gustatory, olfactory, and tactile hallucinations. The user also experiences sensations of weightlessness, macropsia, depersonalization, doubling of the ego (a term for hallucinogenic state), and alteration or loss of time perception. Heffter (42) first suggested that mescaline was the chief constituent of peyote. The potency of peyote depends on the composition of various mescaline-type alkaloids. The chemical formula was determined by Spath (43), and N-acetyl and N,N-dimethyl analogs were isolated from other cactus species that have less psychotomimetic activity than mescaline itself.
been found in virtually all parts of the world. Historically, these hallucinogens are predominately used for producing a mystical experience or a sense of communion with Gods. They were also believed to have special healing powers and to enhance sociability. LaBarre (40) reported that throughout recorded history, hallucinogenic plants were exalted to a sacred role in magico-religious rites. For instance, Aztecs achieved inspiration by eating peyote, called teonanacatl or Gods’ flesh. Similarly, shamans or Indian medicine men of the Rio Grande valley and central Mexico used peyote buttons in their tribal rituals. The earliest report of peyote suggests that the Chichimecas and Toltecs were acquainted with it as early as 300 B.C. (41). Peyote intoxication is characterized by brilliant colored visions in kaleidoscopic movement, often accompanied by auditory, gustatory, olfactory, and tactile hallucinations. The user also experiences sensations of weightlessness, macropsia, depersonalization, doubling of the ego (a term for hallucinogenic state), and alteration or loss of time perception. Heffter (42) first suggested that mescaline was the chief constituent of peyote. The potency of peyote depends on the composition of various mescaline-type alkaloids. The chemical formula was determined by Spath (43), and N-acetyl and N,N-dimethyl analogs were isolated from other cactus species that have less psychotomimetic activity than mescaline itself.
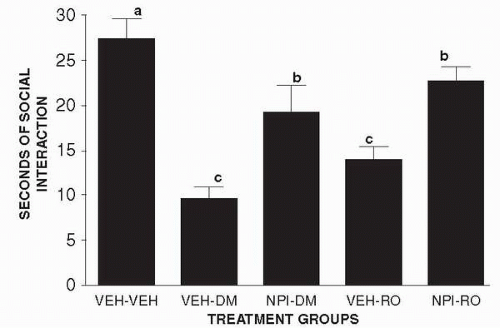 Figure 13.3 • Puerarin and the Social Interaction Test. Effects of pretreatment with puerarin on anxiogenic effects induced by DMCM or Ro 60 0175 in the social interaction test. |
Salvia divinorum (Fig. 13.4) is a sprawling perennial herb which grows wild only in the Sierra Mazatec region of Mexico. It is a rare member of the Lamiaceae (mint) family and has been traditionally used by the Mazatec Indians of northeastern Oaxaca, Mexico, in spiritual practices. Because of its hallucinogenic effects, S. divinorum (also known as magic mint) has been increasingly used as a marijuana substitute in nonethnopharmacologic settings (44, 45, 46, 47, 48). S. divinorum leaf and its leaf preparation, which is occasionally fortified with extracted salvinorin A, are widely available in Western Europe and the United States, notably on Internet sites. In traditional spiritual practices, S. divinorum is typically ingested by one of three routes: chewing and swallowing of the leaves, crushing of the leaves to extract the juices and then swallowing the extract, and smoking the leaves (49). The hallucinogenic effect that results has been reported to be potent and intense, lasting for up to 1 hour (49,50).
 Figure 13.4 • Salvia divinorum.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|