, Götz Penkert2 and Thomas Schelle3
(1)
Neurological Department, Freiberg Hospital, Freiberg, Germany
(2)
Neurosurgical Department, Friederiken-Hospital, Hannover, Germany
(3)
Neurological Department, Municipal Hospital Dessau, Dessau-Rosslau, Germany
9.1 Ilio-hypogastric, Ilio-inguinal, Genito-femoral Nerves
The following chapter about the three inguinal nerves will be short because efforts to expose the small nerves to try neurolysis macro-surgically, or even with a microscope, are useless. We therefore resort to describing anatomical details which we find in visceral surgical descriptions and reports. Nevertheless, there are new therapeutic concepts which can simply be applied to neuropathies as we will later describe in detail.
9.1.1 Damaging Factors
Painful focal nerve lesions of one or more of the inguinal nerves result almost always from hernia surgery. When we read “Hernia Repair Sequelae” in 2010, visceral surgeons worldwide claim about 10–15 % of post-operative painful discomfort [1]. It is interesting that the different authors never distinguish between the different types of groin pain. Local pain syndromes may be due to post-hematoma, fluid effusion or tumor-like fibrotic conditions as a reaction on prosthetic implants. Nerve damage-related groin pain of a radiating character can instead demonstrate various pain qualities as already described in Sect. 4.2. Independent of the type of hernia surgery, herniorrhraphy or hernioplasty with mesh grafts, the rate of painful complications remains the same. Most crucial are post-hernia neuropathic pain cases. Major risk factors related to developing such a pain syndrome are: age below 40 years, pre-existing pre-operative pain, and the male gender. The investigation of mesh explants in order to determine tissue reaction led to the result that younger patients displayed a significantly enhanced infiltration of macrophages after mesh implantation. Older patients were at lower risk of reacting with inflammation on mesh material [1].
Of great importance are, first, an Italian prospective multi-center study done by Alfieri and co-workers in 2006 [2] and second, a previous report by Izard and co-workers in 1996 [3]. Intra-operative identification of all three inguinal nerves during open inguinal hernia repair with or without mesh is reported there to be able to reduce chronic groin pain to less than 1 %.
Although the results of these analyses were published years ago, incapacitating pain syndromes are continuing to occur constantly and frequently. We have therefore to suppose that ligatures or clips for mesh graft fixation or inflammatory tissue reactions are the main causative factors. Industry thus tries to influence surgeons to apply lightweight macro-porous mesh implants to reduce inflammatory reactions on the one hand, and to avoid the possibility of mechanical nerve irritation on the other.
9.1.2 Clinical Symptoms
The symptoms consist of pain, with numbness remaining in the background, weakness is of course absent. The inguinal nerves mainly carry sensory fibers so that, compared to other skin nerves, pain is the leading complaint. It can mostly be manually triggered at the site where substantial damage exists in the abdominal wall. The nerve caliber is so small that intraneural destruction is of a high degree and, consequently, nerve axon regeneration can not be expected. The situation results in neuroma in continuity. The trigger point with radiating pain into the distribution area of the effected inguinal nerve indicates the site of the neuroma. Unfortunately, neuropathic pain types occur frequently. This pain has a burning character; slight touches are perceived as additionally painful (refer to Sect. 4.2). If causalgia occurs, the affected painful skin area is extended, a fact that no longer allows the conclusion which one of the inguinal nerves are really injured. Only the trigger point and the assessment of which skin area the pain then radiates into are helpful.
9.1.3 Electrodiagnostics
There is a significant lack of reports regarding electrodiagnostic testing of the ilio-hypogastric, ilio-inguinal, and genito-femoral nerves in the current literature. Some smaller recent studies or case reports have demonstrated abnormalities in nerve conduction velocities, needle electromyography of the abdominal muscles, reflexology (cremasteric) and somatosensory evoked potentials. All these electrophysiological studies require a technically skilled operator, but are not sufficiently sensitive and specific at the moment [4]. Whether these tests will become routine in general medical practice is uncertain.
Abnormalities in motor nerve conduction using conduction time to the cremasteric muscle, needle electromyography of the cremasteric muscle and cremasteric muscle reflex have been described in 47 % of patients which had undergone herniorrhaphy, whereas the 23 % of patients not treated surgically had problems with the genito-femoral nerve [5]. Similar results could be demonstrated under application of motor nerve conduction studies of the ilio-inguinal nerve [6]. Stand-alone needle electromyography of the abdominal muscles supplied by the ilio-hypogastric and ilio-inguinal nerves revealed signs of subacute or chronic axonal damage in 60 % of patients with definite entrapment and in 37 % with probable entrapment [7]. In future, high resolution ultrasound guided needle electromyography or needle-electrode placement may play a key role in increasing the sensitivity and specificity of these methods. In addition, the registration of somatosensory evoked potentials may be helpful in confirming diagnosis. Therefore, in a single case report the lateral cutaneous branch of the ilio-hypogastric nerve was used for stimulation [8]. Generally, if a unilateral affection is present a side to side comparison of the electrodiagnostic results described above may help and must be closely correlated with clinical findings.
9.1.4 Imaging
High resolution ultrasound provides a simple and cost-effective way to visualize both the ilio-inguinal and ilio-hypogastric nerves [9]. A diagnostic block can be more safely established when guided by ultrasound. It is increasingly important to apply this technique simultaneously, because in general, the diagnosis is assessed on the basis of pain relief within 10 min of local infiltration with anaesthetics [10]. Therefore, the ultrasound probe should be placed with its lateral end just above the anterior superior iliac spine (ASIS) with a perpendicular orientation to the inguinal line. Now the hyperechoic surface of the ASIS and its post-acoustic shadowing is clearly visible laterally. Medially underneath the subcutaneous fat, three layers of abdominal muscles appear (from the outside to the inside): external oblique (EO), internal oblique (IO) and transverse abdominal (TA). Below the transverse abdominal muscle, the peritoneum and the movement of bowels can be observed. Usually the ilio-inguinal and ilio-hypogastric nerves are visible between the layers of the TA and IO muscles, where a splitting of the fascia is normally present (Fig. 9.1b). Nevertheless, there also exist anatomical variants [11]. Sometimes, both nerves can pierce the IO and appear between the IO and EO muscles. They may run together or at a distance of approximately 10 mm. The technique for blocking the genital branch of the genito-femoral nerve under ultrasound guidance has not yet been published [10]. Furthermore, in some of our patients, we were able to demonstrate some pathological changes of the ilio-inguinal and ilio-hypogastric nerves. The most common reactions found included neuroma-formation and enlargement of the cross sectional area (Fig. 9.1a). Until now, major reports on 3 T-MRI of the three nerves are unavailable. In a recent case report on nerve injury locations due to retropubic sling procedures, MRI could not evaluate any abnormalities despite severe chronic pain in symptomatic individuals [12]. Recently Chhabra and Andreisek described the assessment of all three nerves using MRN [13].
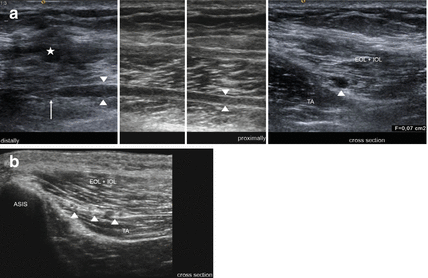

Fig. 9.1
Neuroma formation of the ilio-inguinal nerve after hernia surgery. (a) HRUS shows the stump neuroma (arrow) of the ilio-inguinal nerve (arrowheads) and scarring (asterisk) just above. (b) Normal anatomy. The arrowheads point on the ilio-inguinal and ilio-hypogastric nerves as well as on the deep circumflex iliac artery. ASIS anterior superior iliac spine, EOL + IOL external oblique and internal oblique muscle, TA transverse abdominal muscle
9.1.5 Treatment
Because neurolysis procedures have proved unsatisfactory and, of course, risk in resulting in hernia recurrence, circumscriptive neurotomy is performed worldwide, particularly as so-called “triple neurotomy” in the retroperitoneal space [1].
Neurosurgical pain surgeons have a completely new and comparably simple approach: the idea of pain relief by electrical stimulation of the involved peripheral nerve, a method that has been derived from the “Gate Control Theory” by Melzack and Wall in 1965 [14]. According to their concept, electrical stimulation of fast A-fibers is said to have the capacity to “gate out” pain stimuli conducted via slow C-fibers. It is well known that most pain input to the brain runs via the small unmyelinated and thinly myelinated C-fibers. This input and its transmission to the brain can be reduced by activating of fast running A-fibers. Currently, the concept of nerve stimulation is not only applied to sensory spinal cord fibers but just as successfully to injured peripheral nerves [15]. If the nerve caliber allows piercing of the epineurium, a small electrode is pushed slightly into the sub-epineurial space. We have demonstrated a case with superficial radial nerve branch pain in 2004 [16]. After an external test stimulation period of a few days, the external screener is changed into a pacemaker placed subcutaneously in chest or abdominal wall.
Due to their too small caliber, inguinal nerves do not allow one to advance electrodes into their sub-epineurial space. Experience can now demonstrate that electrodes which were directly beside or in the neighbourhood of nerves did work. With reference to triple neurotomy of hernia surgeons, laparoscopic approaches into the intra-abdominal and retro-peritoneal space were made in order to place electrodes a few centimeters under the peritoneum and beside the affected nerve [17]. The idea was derived from experience with extra-uterine endometriosis-related nerve pain.
Meanwhile, a far simpler approach seems to be the gold standard: so-called subcutaneous peripheral nerve stimulation (sPNS). In local anaesthesia, the electrode is percutaneously advanced near to the location of the assumed nerve injury whereby the trigger point helps to find the site exactly. The intra-operative test stimulation can induce pleasing paresthesias in the distribution area of the affected nerve. After a short external test stimulation period, the pacemaker is – then under general anaesthesia – placed into the abdominal wall about 10 cm from the electrode. This method achieves astonishing responses by the formerly painful conditions as recently reported. In this prospective study of our center, 21 patients are included with a follow up of nearly 10 years. Of these, 76 % had a successful long-term outcome and 24 % had to be considered as long-term failures [18]. Here, after all, sPNS is simple and nearly free of risk, especially if compared with intra- or extra-abdominal approaches. Furthermore, a recent evaluation of 61 spinal cord stimulation patients resulted in the interesting fact that responses did not differ whether the stimulator was used continuously or intermittently [19]. Several of these patients were even proud to have successfully extended their stimulation-out-periods more and more. They stimulated still only facultatively, and after a few years they even requested removal of the complete system. It can be emphasized that responses on post hernia neuropathic pain syndromes are also encouraging but, of course, cases need to be further analyzed.
9.2 Lateral Femoral Cutaneous Nerve
9.2.1 Anatomy
The nerve arises from the L2-root and remains within the retroperitoneal tissue. It exits the pelvis just medial to the anterior superior spine of the iliac crest. The exit point is formed by a split in the lateral attachment of the inguinal ligament at the bone. It then angulates sharply downward, especially in extended hip joint position. The small nerve remains under the fascia and crosses over the upper portion of the sartorius muscle a little to lateral. A few centimeters below the inguinal ligament, the nerve pierces the thick fascia lata. Variations occur frequently [20]. First, the nerve course can be situated more lateral so that it crosses over the iliac bone, second, the level of division into branches varies. It can easily be imagined that a very high ramification makes the exposure more difficult.
9.2.2 Damaging Factors
Most of focal neuropathies of the lateral femoral cutaneous nerve occur spontaneously. The passage between sheaths of the inguinal ligament predisposes the nerve to become compressed or angulated during hip extension. It is often said that symptoms disappear with weight loss, but we also saw underweight patients with the same neuropathy. To summarize, in most cases reliable causative factors cannot be identified. Less common is scarring in the neighbourhood of the nerve responsible for neuralgic pain. It can result from abdominal surgery, open or laparoscopic, and from injury during removal of iliac bone parts as grafts.
9.2.3 Clinical Symptoms
Symptoms are frequently perceived as electric current-like, often suddenly radiating into the distribution area of the nerve of the anterior and lateral aspects of the thigh. The pain does not include the knee and patella region. It is worsened or induced by standing and walking, and relieved by flexion of the hip joint. If the pain history runs long enough, patients complain of additional numbness during painless periods. Numbness unfortunately indicates advanced intra-neural damage within the small nerve, and it worsens the prognosis when neurolysis is done. The electric current-like pain can be provoked by deep manual palpation immediately medial to the anterior superior spine. The same radiating pain disappears if a local anaesthetic is injected at this point. Both of these tests, palpation and injection, serve as clinical proof of the neuropathy of “meralgia paresthetica” as it is usually called. However, this term has to be restricted to those cases with spontaneous onset rather than those related to prior surgery. Those tests also act as an aid to assess the differential diagnosis between a peripheral neuropathy and an L2- or L3-root compression. Both roots have sensory distribution areas similar to that of the lateral femoral cutaneous nerve.
9.2.4 Electrodiagnostics
The lateral femoral cutaneous nerve (LFCN) remained difficult to test reliably by electrophysiological means in the past, likely due to anatomic nerve variability and lack of response in asymptomatic obese subjects [21, 22]. However, recent studies with up to date electrophysiological equipment demonstrate that responses could be obtained in at least 92 % of subjects, even if they are obese [21]. Ultrasound-guided near-nerve needle placement and recording could provide a novel approach, especially when evaluation is critical and responses are difficult to obtain [23]. Orthodromic and antidromic sensory nerve conduction studies are frequently used in the clinical routine. The most appropriate stimulation site in an antidromic nerve conduction study is located 1 cm or more medial to the anterior superior iliac spine (ASIS) or 4 cm distal to the ASIS, whereas sensory nerve action potentials can be simultaneously recorded along an imaginary line between the ASIS and the lateral border of the patella and 2 cm medial to this line [24, 25]. However, normal values are variable and depend on the operator and recording technique. An alternative approach consists of orthodromic nerve conduction study of the lateral femoral cutaneous nerve distally to the ASIS. Of the 120 symptomatic subjects, 98 % had a side to side amplitude ratio greater than 2.3. Combined with the amplitude of the sensory nerve action potential that is lower than 3 μV, this yielded a specificity of 99 % [26]. Furthermore, recording of dermatomal somatosensory evoked potentials provides another option in the diagnosis of meralgia paresthetica. In a recent report, this technique was found to be superior to sensory nerve conduction studies: 81 % vs 65 % sensitivity [27].
9.2.5 Imaging
The lateral cutaneous femoral nerve can easily be identified in the intermuscular space between tensor fasciae latae and sartorius muscles by high resolution ultrasound [28]. Therefore, the sartorius muscle serves as an initial sonographic landmark (Figs. 9.2 and 9.3). Anatomical variations of the nerve course (e.g. piercing of the sartorius) can also be made visible. From this point, the nerve can be traced up to the inguinal ligament and to the anterior superior iliac spine. The cross sectional area of the lateral cutaneous femoral nerve move to behind 1.04 ± 0.44 mm2 [28]. In symptomatic individuals suffering from meralgia paresthetica, high resolution ultrasound was able to show the enlargement of the nerve diameter and cross sectional area [29]. In a recent study the optimal cut-off value for the diagnosis of lateral femoral cutaneous nerve entrapment was 5 mm2 [30]. In our own experience, even the demonstration of different sites of compression (e.g. below the inguinal ligament) with focal congestion of the nerve proximally to them is possible (Fig. 9.2). Moreover, high resolution sonography is not only useful in confirming the diagnosis of meralgia paresthetica but also in ultrasound guided treatment modalities. Whereas conventional techniques (blind or nerve stimulator guided) yielded an incomplete block-success, the ultrasound guided approach was successful in all subjects [31, 32]. Pain relief following application of an anaesthetic confirms the diagnosis at first (Fig. 9.3). After that, one single or two therapeutic perineural injections of 1 mL of methylprednisolone acetate (40 mg/mL) and 8 mL of mepivacaine 2 % can significantly reduce symptoms over a 2-month period [32]. There exist additional case reports on patients treated successfully by means of pulsed radiofrequency ablation, and this may provide in future a relevant therapeutic alternative to surgical interventions described below [33].
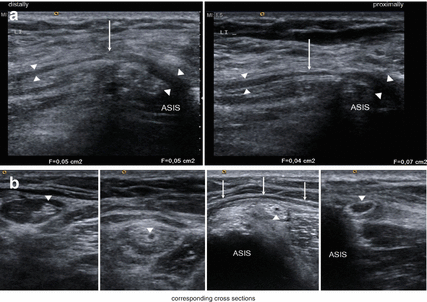
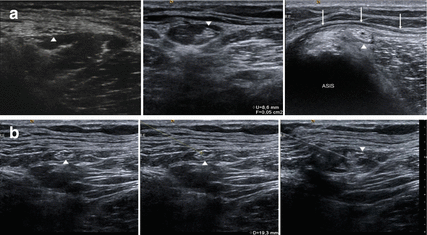

Fig. 9.2
(a) Longitudinal sections of entrapment of the lateral femoral cutaneous nerve (arrowheads) at the level of the anterior superior iliac spine (ASIS) below the inguinal ligament (arrows). Note the proximal nerve swelling. (b) Shows the corresponding cross sections with proximal nerve swelling and effaced fascicles, compression below the inguinal ligament (arrows) and the course of the nerve within tendon of the sartorius muscle and the fat-filled tunnel (from right to left). Arrowheads: lateral femoral cutaneous nerve

Fig. 9.3
(a) Several approaches of ultrasound guided injection of the lateral femoral cutaneous nerve (LFCN, arrowheads): Left: LFCN above the belly of the sartorius muscle. Middle: LCFN within a fat-filled tunnel. Right: LFCN below the inguinal ligament (arrows). ASIS anterior superior iliac spine. (b) Ultrasound guided injection of the LFCN using the “in-plane” scanning technique. Arrowheads: lateral femoral cutaneous nerve
Signal intensity alterations of the lateral femoral cutaneous nerve are difficult to visualize by MR imaging owing to the small size of the nerve [34]. Accordingly, MRI is not the first choice for the diagnosis of lateral femoral cutaneous neuropathy. However, a secondary etiology which is sometimes discussed from e.g. avulsion fractures of the ASIS, sartorius tendon injury, pelvic osteotomy, and acetabular fracture may be demonstrated by MRI [34].
9.2.6 Treatment
If conservative treatment fails (weight reduction, avoidance of tight clothing, drugs acting on neuropathic pain, ultrasound guided injections – see section above) [32], surgery is to be recommended. It consists of nerve release by transection of some parts of the lateral attachment of the inguinal ligament at the iliac bone. It sounds easy, but difficulties may occur with the small caliber of the nerve and its branches and with the above-mentioned anatomical anomalies. Therefore, repeated injections with corticosteroids or ultrasound-guided radiofrequency ablation are used and are also reported to be successful [32, 33]. Unfortunately, however, pitfalls of injection therapy trials can have a worsened prognosis if surgical neurolysis is in the end intended. First, the numbness is progressed, and, second, direct nerve substance damage can be caused by needles or injected substances. The prognosis is especially deteriorated when the character of the pain changes into a neuropathic one. We therefore prefer early surgery and perform it as neurolysis. We avoid a neurotomy so as not to risk a painful neuroma as a result (Fig. 9.4).
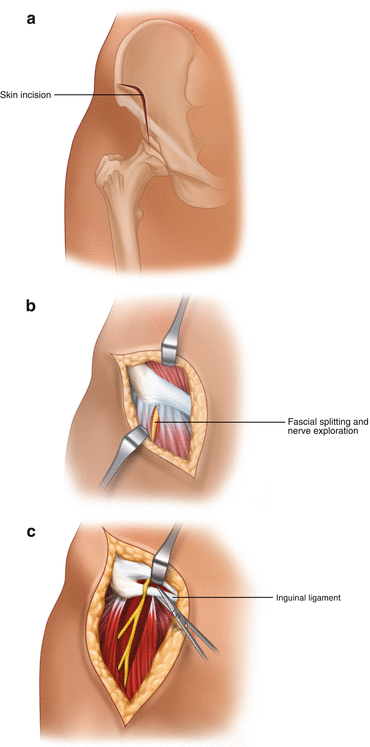

Fig. 9.4
Surgical treatment of “Meralgia paresthetica”. (a) Skin incision. (b) First step: nerve exposure by splitting of the ventral limb fascia medial to the sartorius muscle. (c) Second step: nerve exposure to proximal aiming at transection of transverse inguinal ligament parts particularly inferior to the nerve
We personally prefer the approach which starts below the inguinal ligament. The skin incision is done anterior and a little superior of the spine and it then runs straight downward over a distance of about 4–5 cm. Next the fascia of the thigh is incised longitudinally. Beside the medial border of the sartorius muscle, the cutaneous nerve has to be identified as embedded in fat tissue; parallel to the nerve or its branches, a small venous vessel helps to find the thin nerve. After careful identification of the nerve or two or even three of its branches, we advance to proximal until the above-mentioned split between two parts of the inguinal ligament becomes visible. It is more important to transect the part which crosses under the angulated nerve than the part which covers the nerve course. During ligament fiber transection, the small nerve should be gently kept away a little. Particularly, if small bleedings appear at this moment, bipolar coagulation requires the nerve to be kept away. Surgical results of this approach are formally published [35].
As an alternative, some authors use a supra-inguinal approach which can facilitate nerve identification proximal to its ramification [36]. It needs the help of an assistant who keeps a retractor to medial manually whereas the infra-inguinal approach can be accomplished with self-holding retractors.
As we have experienced early recurrences following the surgery as outpatient management, we always advise hospitalization for a few days, and a suction drain to remain for no less than 3 days to avoid any re-bleeding or lymph fluid pad which likely occur in particular when the patient does not remain immobilized for long enough.
Pitfalls and pain recurrence depend on different factors, of course; on the efficiency of the inguinal ligament fiber transection, but also on the amount of intraneural damage that happened preoperatively, and, not to be neglected, on the preoperative pain type. Cases with hyperesthesia or even allodynia have a worse prognosis, whereas cases with a pain that can be triggered, and at the same time is without numbness, have the best prognosis. Injury to one of the small nerve branches can be followed by remaining neuropathic pain involving its small skin distribution, as experienced in rare cases.
9.3 Obturator Nerve
Focal neuropathies of the obturator nerve may commonly result from intra-pelvic trauma or surgery. As an intra-pelvic nerve exposure with neurolysis or proximal neurotomy seems to become unsatisfying, as described in comparable cases of inguinal nerve lesions in Sect. 9.1, we will neglect these almost incurable lesions in the following. However, several interesting experiences with spontaneous neuropathy exist, and they shall be covered in this chapter. Literature is rather lacking concerning these lesions.
9.3.1 Anatomy
The obturator nerve originates from the lumbo-sacral plexus (roots L2–L4). These are the same roots which contain all the fibers to the femoral nerve. It runs downward at the medial border of the psoas muscle, and then takes a vertical course to pierce the obturator membrane. At extra-pelvic level, the nerve at once divides into two branches, an anterior or superficial branch which supplies the adductor longus and gracilis muscles, and a posterior or deep branch, which supplies the adductor magnus and obturator externus muscles. Some sensory fibers represent sensation in a small skin area on the medial aspect of the thigh.
9.3.2 Damaging Factors
The possible relation of nerve injury to pelvic fractures or hip surgery has already been mentioned. Intra-pelvic malignancies may invade the nerve. Obturator hernias can involve the nerve. Newborns can suffer from obturator palsy; but they usually recover quickly [37]. Reliable factors which cause a compression syndrome with spontaneous onset are quite unknown. We could gain experience with the relatively easy exploration of the nerve’s exit site where the extra-pelvic course starts. We would like to emphasize the point that the split between obturator membrane and margin of the superior pubis bone branch is the location of a focal “spontaneously occuring” entrapment.
9.3.3 Clinical Symptoms
A small skin branch distributes a circumscriptive area at the medial and proximal aspect of the thigh. Pain is located there in the case of nerve entrapment. Numbness indicates a severe lesion and is therefore restricted to intra-pelvic nerve damage. Pathological potentials in the adductor muscles may be found in electromyography testing (see below). The diagnosis mainly consists of an infiltration with local anaesthesia at the point where we can elicit the pain by deep palpation, namely through the pectineus muscle. This test also helps to differentiate the obturator neuralgia from genital branch neuralgia.
9.3.4 Electrodiagnostics
Literature is rather sparse concerning nerve conduction studies of the obturator nerve and therefore limited in collaborative electrophysiological data. None of the few reported techniques on the subject are used routinely. A distal motor latency can be obtained by stimulating the obturator nerve at the inguinal ligament, while M-responses were recorded with needle electrodes from the gracilis muscle. An additional stimulation point – using needle electrodes at the level of the upper lumbar roots between L1–L2 vertebral laminae – generates a proximal conduction time [38]. In healthy individuals, the following normal values were reported: distal motor conduction latency 3.9 ± 0.7 ms, proximal conduction time 10.4 ± 0.3 m, and conduction velocity of the proximal segment 62 m/s [38]. An alternative method consists of magnetic stimulation of the paralumbar area, where the M-responses are recorded via needle electrodes placed in the adductor longus and adductor brevis muscles [39]. However, needle electromyography still remains the key method of evaluating obturator nerve function preexisting axonal damage. Due to the assessment of muscles only supplied by the obturator nerve, of muscles innervated by adjacent nerves, and of paraspinal muscles, a differentiation between an isolated obturator nerve neuropathy, lumbar plexopathy, or L3-root affection becomes feasible [40].
9.3.5 Imaging
High resolution ultrasound is of subordinate importance in the diagnosis of obturator nerve neuropathy, because only the extra-pelvic nerve course can be assessed. The femoral vein and artery below the inguinal ligament serve as a landmark; by moving the probe medially and caudally to the pectineus muscle and then to the adductor muscles (consisting of the adductor longus, brevis and magnus from cranial to caudal) the nerve can easily be identified. The anterior branch of the obturator nerve with the adjacent vessels can be found in the fascia split between the pectineus, adductor longus and adductor brevis muscles, whereas the posterior branch with the accompanying vessels appears in the fascia split between the adductor brevis and magnus muscles. The following normal values (anterior-posterior diameter) have been reported so far: Anterior branch 1.4 ± 0.6 mm, posterior branch 1.7 ± 0.6 mm, main trunk 2.7 ± 1.2 mm [41, 42]. Tilting the probe cranially, the main trunk may be visible in slim individuals (Fig. 9.5). This technique is in use for ultrasound guided obturator nerve blocks [43]. As mentioned in the previous chapter, pain relief after infiltration with local anaesthetics again supports the diagnosis [40]. However, sonographical reports about extra-pelvic affections of the obturator nerve are missing at the moment. In contrast to that, 3 T-MRI allows the assessment of the entire course of the obturator nerve: the intra- and extra-pelvic parts. Particularly, by means of MRI, space-occupying lesions such as hematoma, bone fragments, bursa, nerve sheath tumor, malignancies, or osteo-synthesis materials as well as fat infiltration just proximal to and within the obturator canal can be assessed. On the other hand, muscle substance alternation related to acute or chronic denervation may be documented [34].
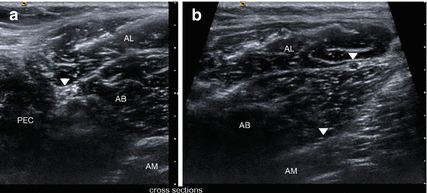

Fig. 9.5
(a) Normal cross-sectional anatomy of the extra-pelvic course of the obturator nerve in HRUS. The main trunk (arrowhead) appears caudally between pectineus (PEC), adductor longus (AL) and adductor brevis (AB) muscles. (b) More distally the anterior branch (arrowhead) appears in the fascia split between adductor longus (AL) and the adductor brevis (AB) muscles whereas posterior branch (arrowhead) can be located in the fascia split between adductor brevis (AB) and adductor magnus (AM) muscles
9.3.6 Treatment
In the following, we restrict ourselves to describing the approach to the easily accessible extra-pelvic nerve part, the nerve segment, which may be involved in the sense of focal entrapment. We start with a skin incision parallel to the medial segment of the inguinal ligament and extend the incision vertically downward over 4–5 cm. The incision allows an approach between the long adductor muscle and the pectineus muscle. Of course, fibers of the pectineus muscle can also be split by blunt dissection. Underneath the layer of these two muscles, we enter a flat layer of fat tissue in which the two mentioned branches of the obturator nerve are embedded. These nerve branches and the concomitant obturator artery and its branches are gently isolated, and next we follow to proximal in order to find the exit point through the obturator membrane. By means of a small punch, the lower margin of the ramus superior ossis pubis is opened in an inverse U-shape always with the exiting nerve and artery in view. The only literature concerning this approach we believe to be available is from Millesi from 1992 [44]. However, interestingly his contribution does not consider spontaneous entrapment cases at all (Fig. 9.6a, b).
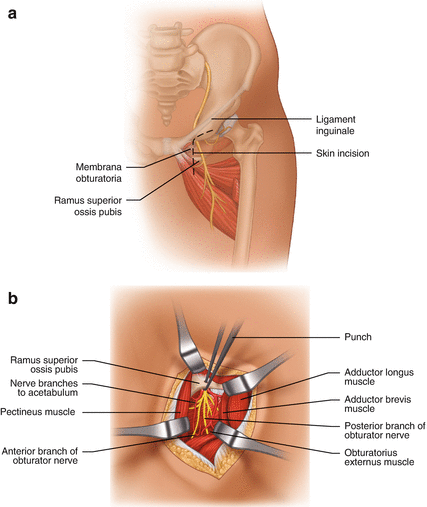

Fig. 9.6
Surgical treatment of obturator nerve entrapment. (a) Skin incision. (b) Nerve exposure via split pectineus muscle fibers; the bony roof of the nerve’s exit is opened by a punch
9.4 Sciatic Nerve
A focal entrapment of the sciatic nerve can rarely occur within the infra-piriforme foramen. Birch specifies the historical literature which we can find dealing with the so-called “piriformis syndrome” [45]. The term is controversial in that a lot of pain syndromes in neighbourhood of the hip joint are included by different authors. In the following, we restrict ourselves to applying this term to cases which are severely associated with pain radiating under the foot, indicating a sciatic nerve irritation.
9.4.1 Anatomy
The sciatic nerve leaves the pelvis together with the inferior gluteal nerve and vessels through the lower part of the great sciatic foramen. The foramen is divided into higher and lower openings by the transverse running piriformis muscle. Its tendon attaches at the great trochanter of the femur. Sometimes, the tendon is extended more than normal to medial so that a fibrotic structure crosses over the sciatic nerve. As anatomical variation, the nerve may be divided into two trunks with the piriformis muscle running between both. In these cases, the superior trunk leads fibers running into the peroneal nerve distribution whereas the lower trunk contains fibers of the tibial nerve. A surgeon should be aware of this variation which can frequently occur.
9.4.2 Damaging Factors
Completely comparable to the thoracic outlet syndrome, where fibrotic structures within the scalene muscles cause plexus irritation, here a fibrotic and too medially extended tendon within the piriformis muscle leads to discomfort. It is not the muscle belly itself, but its tendon. Small persons with little buttock substance are predisposed. Three times we have observed patients who always used to keep their purse in the same back pocket while sitting in a car or on a bicycle. They instinctively left it out sometimes and the pain progressively disappeared. Another time, we were confronted with a patient who presented with a subtotal foot drop and additional weakness of the plantar flexion on both sides after he had been confined to bed in a supine position for 2 weeks in an intensive care unit. On the other hand, cases with spontaneous onset or without any known cause have required us to find a solution.
Magnetic resonance imaging (MRI) has, of course, to exclude space-occupying lesions within the pelvic area. The main differential diagnosis is commonly a lumbar disk protrusion; the sign according to Lasegue is always pathognomic in root compression whereas likely to be negative in the case of a “piriformis syndrome”, but, unfortunately, also negative in the case of a narrowed root recess.
9.4.3 Clinical Symptoms
The patient suffers from electric current-like pain which radiates into the tibial and peroneal nerve distribution either spontaneously, or when we palpate the gluteal area deep enough and just medial to the hip joint. A palsy of foot extension and/or flexion appears rarely, but has to be taken into account as mentioned above.
Computerized tomography- or ultrasound-guided test injections can be used to differentiate between sciatica due to either spinal root compression or piriformis irritation: A nerve root infiltration influences the afferent input from both levels, from spinal and infrapiriforme ones, whereas an infiltration into the infrapiriforme foramen cannot influence afferent fibers which are compressed superiorly; thus, a positive effect in the periphery excludes a spinal root compression. Of course, magnetic resonance imaging (MRI) of the thoraco-lumbar spine and the pelvis is mandatory to use all possible means to exclude sciatica of alternative origin.
9.4.4 Electrodiagnostics
Electrodiagnostic testing may be of some assistance in determining nerve irritation. The sciatic nerve is stimulated proximally at the gluteal fold using a 75-mm monopolar needle, and distally at popliteal level, the peroneal and tibial nerves being stimulated separately. The recording electrodes are positioned over the extensor digitorum brevis and abductor hallucis muscles for examining the peroneal and tibial portions of the sciatic nerve. Motor nerve conduction velocities (MNCV) are, for the tibial nerve division 52.8 ± ms and, for the peroneal nerve division 54.3 ± 4.4 ms [46]. MNCV of the sciatic nerve can be measured at the gluteal segment by magnetic stimulation, proximally at L5 and S1 roots and distally at the sciatic nerve at the gluteal fold followed by recording at the corresponding muscles. The mean MNCV of the gluteal nerve segment in the L5 component was 55.4+/−7.8 m/s in patients with piriformis syndrome, which was slower than the mean value of 68.1+/−10.3 m/s obtained in healthy controls. The MNCV of the sciatic nerve in the S1 component showed no significant difference between patients and controls. A negative relation was found between the disease duration and the MNCV values in piriformis syndrome. It was concluded that magnetic nerve stimulation provides a painless, non-invasive and objective method to evaluate sciatic nerve function in patients with piriformis syndrome [47].
In clinical practice, however, commonly the electrodiagnostic studies include the sensory study of sural and further superficial peroneal nerves, and, of course, the motor studies of the peroneal and tibial nerves (see below). Abnormal SNAPs of sensory nerves imply an infraganglional lesion. H-reflexes and F-waves are unable to localize the lesion focus. Needle electromyography is the most informative in sciatic nerve lesions. Gluteal sciatic nerve irritation – such as in piriformis syndromes – can demonstrate slightly pathologic findings in semitendinosus and semimembranosus muscles, in the long head of biceps femoris, and in lower leg muscles [48].
9.4.5 Imaging
As previously mentioned in Sect. 6.1.3, the sciatic nerve course can be visualized well by means of high-resolution ultrasound distally of the gluteal fold, whereas the intrapelvic and gluteal regions hardly permit to obtain reliable information in ultrasound (Figs. 9.7, 6.8e and 6.22a).
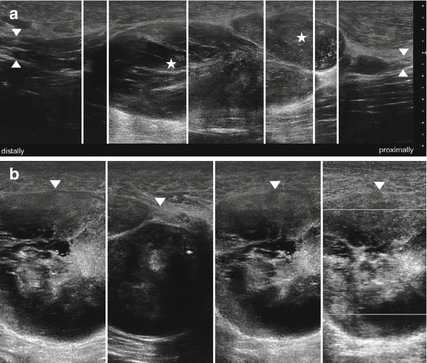

Fig. 9.7
(a) HRUS, longitudinal section of the sciatic nerve (arrowhead) demonstrating multiple large neurofibromas (asterisk) in a patient with neurofibromatosis type I. (b) Cross sections. The tumor matrix (arrowheads) shows no vascularization as well as regressive changes
The method of choice is MR neurography because the sciatic nerve is easily evaluated in all imaging planes due to its large size and abundant perineural fat (Figs. 6.21 and 6.22b). The nerve has intermediate signal intensity on T1-weighted images and mildly high signal intensity on fluid-sensitive images. It can be followed as it descends in the greater sciatic foramen and exits the pelvis under the inferior margin of the piriformis muscle. Direct MR imaging evidence of sciatic neuropathy includes course deviation, increased size and increased signal intensity. The latter is more easily detected than in lower extremity nerves owing to the large size of the sciatic nerve. Obliteration of the fat planes around the nerve trunk may also be noted [34]. However, imaging diagnosis of piriformis syndrome is problematic. Although the diagnosis can be commonly inferred from changes in the appearance of the affected nerve, evaluation of piriformis muscle size and its comparison with the contralateral side are less than satisfying, as muscle anomalies and significant variations in size are noted in symptomatic and asymptomatic individuals [34]. In a review of 100 patients with no history or clinical suspicion of piriformis syndrome, muscle size asymmetry of 2 mm was present in 81 % of patients. None of the patients with asymmetry of 4 mm or more had symptoms suggestive of piriformis syndrome [49].
Using MR neurography it was found that, of patients who responded well to piriformis surgery, 38.5 % had ipsilateral muscle hypertrophy and 15 % had muscular atrophy. Muscle asymmetry alone turned out to have a specificity of 66 % and sensitivity of 46 %, identifying patients with muscle-based piriformis syndrome. Conversely, ipsilateral nerve edema was 88 % associated with reproducible symptoms of piriformis syndrome. Use of both asymmetry of the piriformis muscle and increased nerve signal intensity improved the diagnostic ability of MR neurography, with 93 % specificity and 64 % sensitivity [50]. In a retrospectively reviewed MRN study of the sciatic nerve in the pelvis and thighs of 34 subjects, of which 17 had a final diagnosis of sciatic neuropathy according to electrodiagnostic or surgical confirmation, it was found that sciatic nerves of the affected sides exhibited higher nerve-to-vessel SI (signal intensity) ratios and, furthermore, higher incidences of T2 hyper-intensity, enlargement, and abnormal fascicular shape compared to sciatic nerves of the normal side. A cut-off value of nerve-to-vessel SI ratio of 0.89 exhibited high sensitivity and specificity in predicting sciatic neuropathy. In particular, calculation of the nerve-to-vessel SI ratio demonstrated excellent reliability. The authors conclude that both qualitative and quantitative criteria should be used to suggest the MRN diagnosis of sciatic neuropathy [51]. Nevertheless, most authorities agree that delineation of the neuromuscular relationship will turn out to become the most important goal of imaging. Until reproducible and reliable criteria have been established, piriformis syndrome will remain a diagnosis by exclusion [52].
9.4.6 Treatment
CT- or ultrasound-guided botulinum toxin injections into the piriforme muscle belly are performed repeatedly with success. However, the first surgical approach was described by Robinson in 1947 [53]. He sectioned the piriformis muscle a few centimetres beside its attachment at the great trochanter completely. He chose a lateral transection in order to assure the preservation of all major vessels which lie concomitant to the sciatic nerve and its branches. The same author describes several anatomical variations as existent in 10 % of specimens, e.g. the division of the sciatic trunk.
We prefer surgery and choose a skin incision parallel to the expected fibers of the gluteus maximus muscle; then the muscle fibers are split and separated to both sides and manually retracted. Fibers of the gluteus medius muscle which are situated underneath can be lifted up and also retracted. The sciatic nerve trunk can be felt medial to the hip joint as a longitudinally downward running structure of index finger diameter. It is embedded in fat tissue and accompanied by the inferior gluteal artery and a venous plexus. The dissection to proximal requires care to preserve as much of this plexus as possible. Transverse vessels have to be visualized before rupture, closed by bipolar coagulation far enough from nerve fibers, and finally transected. Vessel rupture immediately colours the operating field black. The sensory posterior femoral cutaneous nerve branch which runs downward and medial to the sciatic nerve has to be preserved; its injury can result in numbness or even neuropathic pain at the back of the thigh and in the perineum via a small perhaps not unimportant nerve branch. By proceeding to proximal, the transverse running fibrotic lower edge of the piriformis should come into view, and it then requires transection piece by piece above the exiting nerve. However, before doing so, the inferior gluteal nerve should be visualized; it curves around the lower edge of the piriformis and then upwards. The muscle belly itself can of course be preserved, comparably to the situation in the scalene notch, as muscle fibers do not irritate nerve structures at all.
It may rarely happen that a large venous convolute occurs within the infrapiriforme foramen on the left side. This rare phenomenon is related to the possible venous “pelvic congestion syndrome” which can occur by a so-called “nutcracker-phenomenon” – a compression of the left iliac vein between lumbar spine and left iliac artery or of the left renal vein between aorta and superior mesenteric artery [54, 55].
As it is almost impossible to preserve the whole venous plexus during surgery, a deeply advanced suction drain of large caliber is mandatory to avoid hematoma-complications, especially when the patient gets up. As a final remark concerning our indication, surgical treatment is only being considered when typical electric current-like radiating sciatica is present. Our goal cannot be anything else than decompression of a focal nerve entrapment. Pain syndromes which instead remain in the buttock – e.g. inflammatory tendopathies – do not offer any surgical option at the present time.
9.5 Pudendal Nerve
9.5.1 Anatomy
The pudendal nerve arises from the second, third and fourth sacral nerve roots, leaves the pelvis through the great sciatic foramen, and re-enters the pelvis through the lesser sciatic foramen. The fibrous canal between both foramens is referred to as the Alcock canal; therefore, neuropathies of the pudendal nerve were described as Alcock’s canal syndrome [56]. The nerve supplies half of the perineum and genital region with sensory fibers. Its focal entrapment evokes intractable pain attacks in this area. The clinically important fact that the perineum is also supplied by a small branch of the posterior femoral cutaneous nerve should never be neglected (Fig. 9.8a–d).
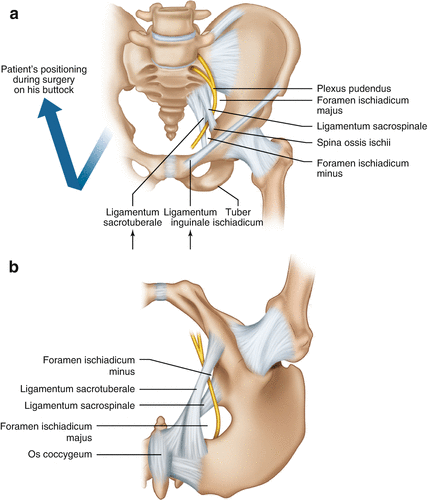
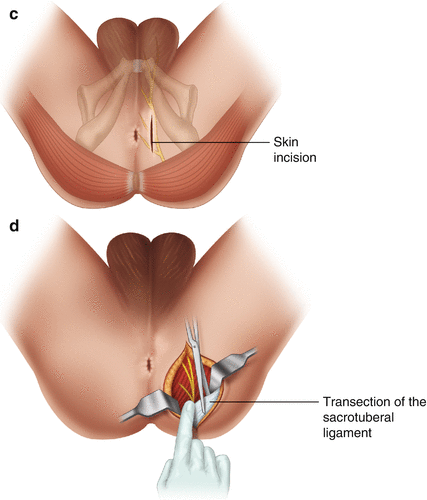


Fig. 9.8
(a) Anatomy of the pudendal nerve course. (b) Anatomy after patient’s positioning on his back with lifted legs; nerve’s relation to sacrospinal and sacrotuberal ligaments. (c) Skin incision. (d) Nerve exposure and transection of the sacrotuberal ligament under the gluteus maximus muscle fibers; the ligament is, if existent, to be identified by your fingertip
9.5.2 Damaging Factors
Of course, trauma relation or iatrogenic damage and space-occupying lesions can affect the nerve. However, on the other hand, prolonged bicycle rides have been already repeatedly described [57]; similar histories have also been observed by us. This mechanism should be assessed as the main causative factor when a neuropathy seems to appear spontaneously. The nerve is then trapped between the ischial spine and the sacro-tuberous ligament. In particular, the falciforme lower edge of the ligament is able to pinch the nerve if pressure arises between body and bicycle saddle. A figure in Steward’s book gives us a reasonable impression of this entrapment site [58].
9.5.3 Clinical Symptoms
Patients describe unilateral, electric current-like, pain within the genital region and perineum with sudden onset comparable to the “tic doloureux” in a trigeminal neuralgia. Only one of our patients –a small person with little buttocks – suffered from alternating pain attacks on both sides.
A more or less continuous pain which remains restricted to the perineum might be of another origin: This restricted pain area can likely belong to the distribution of the perineal (cluneal) branch of the posterior cutaneous nerve, as mentioned in Sect. 9.4.
When the patient lies supine with his hip joint 90° flexed so that the leg stands vertically, the typical Tinel sign can be elicited about three fingertips beside the anus. It indicates the lower edge of the sacro-tuberous ligament. A careful test infiltration – perhaps ultrasound-guided – with local anaesthesia at this point removes the pain attacks for 2 h and creates a genital numbness instead. In a recent paper, these nerve blocks even resulted in therapeutic effects in 44 % of cases [59]. Only patients who satisfactorily responded to our test infiltration at the described site were selected for a small surgical decompression.
9.5.4 Electrodiagnostics
According to the anatomical course of the pudendal nerve, several sites of compression can lead to various clinical presentations of pudendal neuropathy [60]. As mentioned in Chap. 5, the unmyelinated C-fibers, carrying pain sensation are not assessable with conventional nerve conduction studies, whereas fecal and urinary incontinence, impotence and numbness of the skin area supplied by the nerve thereby may be relevant. In the past two decades, different electrodiagnostic techniques have been introduced, but most of them are not in general use. Needle electromyography of the anal sphincter, registration of terminal motor latency of the pudendal nerve after transrectal electrical stimulation, as well as somatosensory evoked potentials of the pudendal nerve may be helpful for making a diagnosis. In particular, a prolonged terminal motor latency and signs of anal sphincter denervation in needle electromyography suggest an involvement of the anal motor nerve, whereas changes in somatosensory evoked potentials of the pudendal nerve may verify a sensory neuropathy [61]. However, in 2008, a group of experts published five essential (exclusively clinical) diagnostic criteria for pudendal neuralgia by focal nerve entrapment (“Nantes criteria”): “(1) pain in the anatomical territory of the pudendal nerve; (2) worsened by sitting; (3) the patient is not woken at night by the pain; (4) no objective sensory loss on clinical examination; (5) positive effect by anaesthetic pudendal nerve block” [62]. In this set of criteria, electrodiagnostic testing plays only a subordinate role. We have unfortunately to realize that our electrodiagnostic techniques do not differentiate between entrapment and alternative nerve lesion (especially obstetrical damage in uni- or multiparous women). Therefore they do not give any direct information about pain etiology. Consequently, they have a rather limited sensitivity and specificity. In fact, they might perhaps be helpful before surgery in order to assess the quality of motor innervation of the nerve, and they might predict the outcome [63].
9.5.5 Imaging
In a recent report it has been shown that high resolution ultrasound can identify the pudendal nerve at the ischial spine when using a medial approach, and its terminal branches when using an anterior approach [64]. Unfortunately, its value in the diagnosis of different pathological processes involving the nerve, especially if entrapment is supposed, remains unclear at the moment. Until now, pain relief after blocking the pudendal nerve with anaesthetics is the superior diagnostic criterion in pudendal neuralgia [62]. Sonography may also be applied for ultrasound guided nerve blocks. Using colour Doppler to localize the internal pudendal artery at the ischium, in a second step, the sacro-spinous and sacro-tuberous ligaments have to be targeted. The pudendal nerve may be found in the plane between these two ligaments [10]. Regarding pudendal nerve entrapment and MR imaging, it was recently reported that the neurovascular bundle can be visualized by MR neurography between the ischial spine and the Alcock canal. In the case of entrapment, it is reported as typical to find asymmetric nerve swelling and hyperintensity on T2-weighted and fat-saturated images [65].
9.5.6 Treatment
Therapeutic concepts extend from repeated steroid injections via trans-vaginal approach, CT-guided trans-perineal injections [66], surgical decompression via trans-perineal approach [67], trans-gluteal approach with exposure of the entire Alcock’s canal [56], until neuro-modulation via electrodes on sacral nerve roots [68] or even on the spinal cord [69]. Of course, we have first to consider that a disorder of the pudendal nerve distribution can present a wide field of individual symptoms, and, second, that it is commonly combined with progressed social disintegration. Therefore, dedicated therapeutic approaches and options can only be offered where centers deal with all types of pudendal neuralgia [70]. Particular difficulties arise when the pain has achieved neuropathic character. Our own experience is exclusively restricted to those cases which could be manually triggered at the lower edge of the sacro-tuberous ligament, and which could be tested there by local anaesthetics.
The surgical approach to this focal entrapment site is very simple. It can be easily derived from anatomical figures [72]. The patient’s positioning is comparable to that needed in proctologic approaches. The skin incision is done approximately 3 cm beside the anus and a little bit dorsal to respectively below it. The lower edge of the gluteus muscle fibers is separated from fat tissue. At this point, the trapping ligament underneath the muscle should be palpated with a fingertip. In a few operated cases, the ligament as a solitary structure was absent, but instead, several transverse running fibrous bands could be palpated. The pudendal nerve exits upon the ligament or the fibrous bands when we bring to mind the patient’s position in the operating theatre. Identification of the pudendal nerve is not as easy as expected because it is embedded in abundant fat tissue and therefore difficult to survey. However, consideration of the pre-operatively discovered trigger point in relation to the position of the anus finally helps to isolate the nerve. The last surgical step is partial or complete transection of the sacro-tuberous ligament where it covers the nerve’s exit (Fig. 9.8c–d).
As mentioned, several specialized centers exist worldwide where experts of different disciplines work together in the field of pudendal neuropathy. However, around 60 % of these cases are suitable for trying the simple approach described above [70]. Our own experience is rather small, but at least it proved successful provided we remained restricted to neuralgias which originated from the nerve’s exit zone. This chapter shall not in any case cover the whole entity of pudendal neuropathies.
9.6 Femoral Nerve
The femoral nerve is never affected by a spontaneously occurring irritation or entrapment only. Pre-formed anatomical structures like tendons, ligaments or a narrowed channel which could irritate the nerve in a sense of focal neuropathy do not exist. Therefore, our description remains short.
9.6.1 Anatomy
The femoral nerve arises from the L3 and L4 spinal roots, and it then starts downward at the lateral aspect of the psoas muscle. Its course is covered by the iliacus fascia. Under the inguinal ligament it exits the pelvis lateral to the femoral artery and vein. Then it divides at once into several motor branches which supply the quadriceps muscles, and into sensory branches which innervate the skin of the ventral thigh medial to the area of the lateral femoral cutaneous nerve. One of its branches – the saphenous nerve – will be the subject of the next main section.
9.6.2 Damaging Factors
We have already mentioned that entrapments of focal character with spontaneous occurrence do not exist. Nontheless we once tried a single neurolyisis under the inguinal ligament in a patient with progressive loss of quadriceps muscle strength without success. The young man probably suffered from one of the inflammatory disorders we will describe in Chap. 12.
Reasonable femoral nerve impairments are instead the following: penetrating injuries in the groin, or below, at the thigh, lesions in association with pelvic fracture, inguinal node resection, femoral artery puncture, all kinds of intra-pelvic surgery, hip arthroplasty, hematoma or abscess beneath the iliacus fascia, false aneurysms following rupture of an aortic aneurysm, malignancies of the iliac bone or other space-occupying lesions like harmless cysts or ganglia. In 1934, anatomists described those rare cysts as originating from occasional communications with the hip joint [72]. In addition, in 1982, a possible way to evaluate these rare lesions by ultrasound was published; the paper again indicates that this type of investigation is easily applicable [73]. Therefore, in general, if a femoral neuropathy occurs, imaging seems mandatory to discover the reason before surgery is considered.
9.6.3 Clinical Symptoms
Patients always suffer from loss of muscle strength of the quadriceps. The disability more or less keeps the knee joint extended and impairs patients’ standing and walking. Numbness and paresthesias are not the main complaints, but rather the weakness. The knee reflex can be absent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







