5 Neurinomas of the Vestibular Nerves
 Basic Remarks
Basic Remarks
 Surgical Management of Acoustic Neurinomas
Surgical Management of Acoustic Neurinomas
Basic Remarks
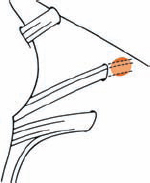
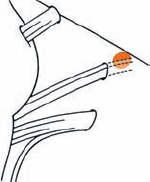
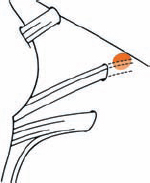
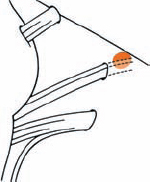
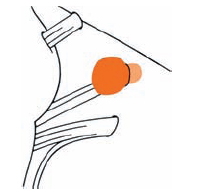
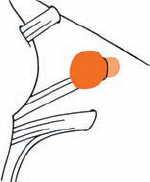
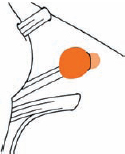
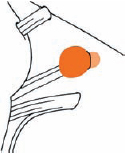
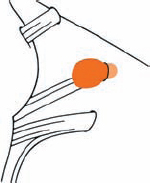
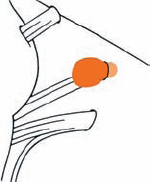
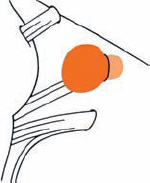
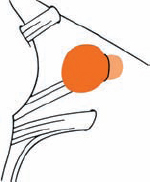
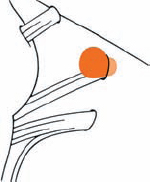
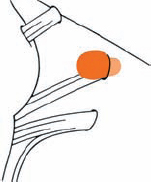
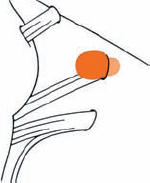
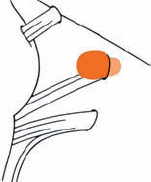
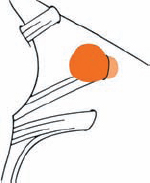
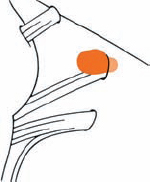
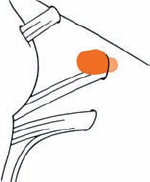
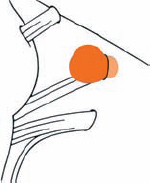
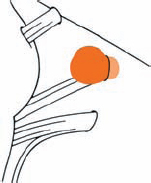
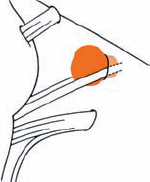
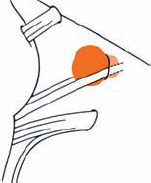
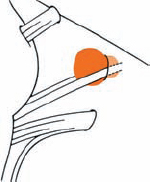
Tumor Grading by Size (Figs. 5.1, 5.2)
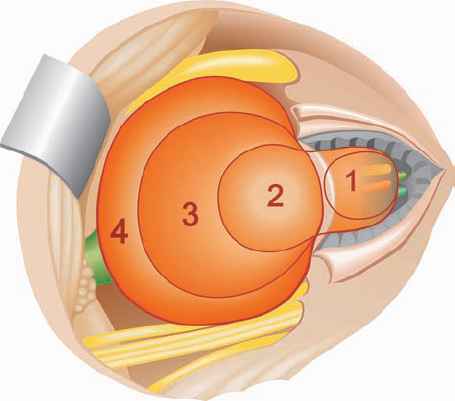
Fig. 5.1 The grading system for these tumors is included here, as the techniques and results vary markedly between different tumor sizes.
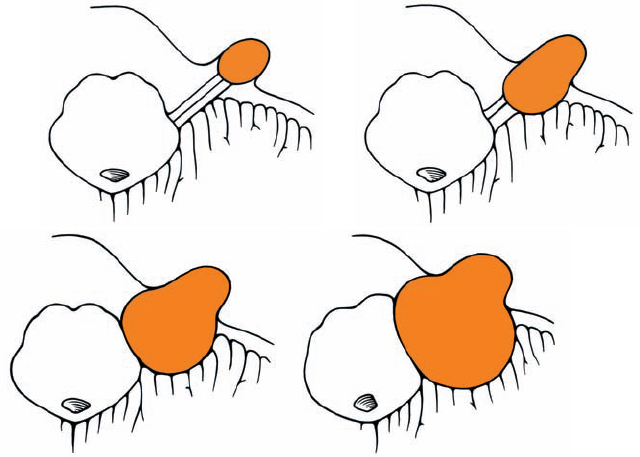
Fig. 5.2 Grade 1: intracanalicular tumor, with a longitudinal diameter of 1–10 mm. Grade 2: intracanalicular and intracisternal tumor, with a longitudinal diameter up to 20 mm. Grade 3: Intracanalicular and intracisternal tumor, with a longitudinal diameter up to 30 mm (reaching the brain stem). Grade 4: intracanalicular and intracisternal tumor, with a longitudinal diameter more than 30 mm (indenting and displacing the brain stem).
Origin and Growth Pattern of Acoustic Neurinomas (Figs. 5.3–5.5)
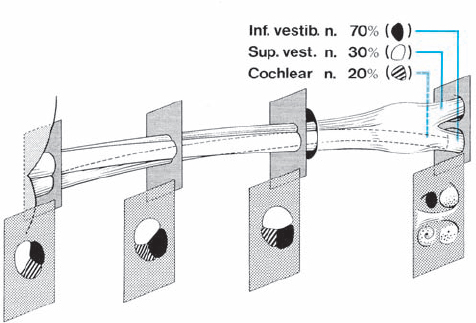
Fig. 5.3 The origin of acoustic neurinomas. At the fundus of the internal auditory canal, the inferior vestibular nerve is consumed by tumor in more than 70 % of cases, the superior vestibular nerve in 30 %, and the cochlear nerve in less than 20% of cases. In the majority of cases, “acoustic neurinomas” originate from the vestibular portion of the eighth nerve in its intrameatal course, but may also involve other components of the eighth nerve. All of these tumors originate in the neurilemmal segment of the nerve, lateral to the glial–neurilemmal junction.
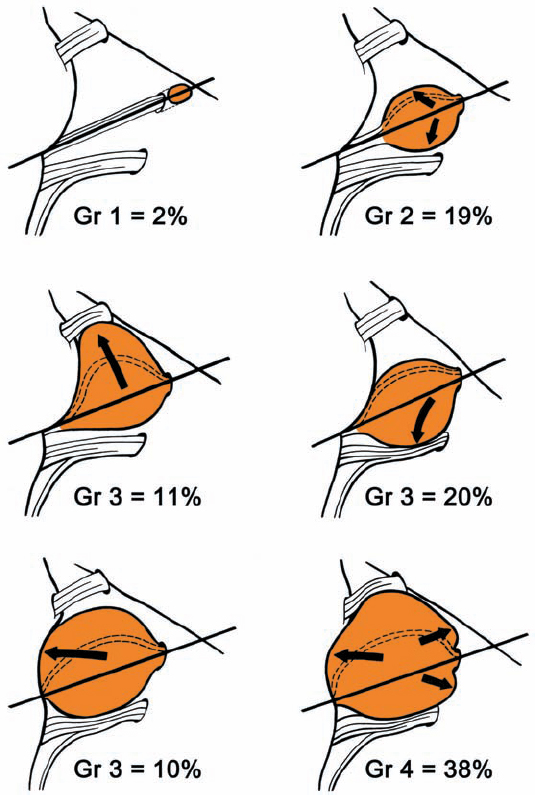
Fig. 5.4 Growth patterns of acoustic neurinomas in relation to tumor grades.
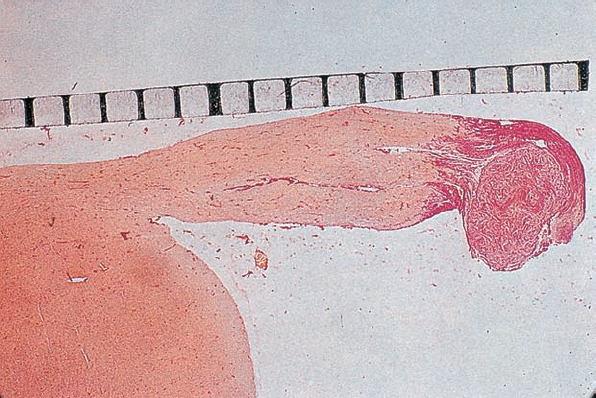
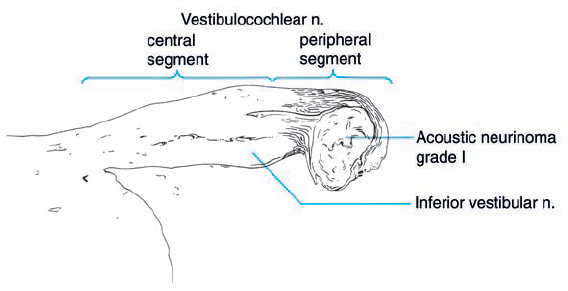
Fig. 5.5 A histologic section demonstrating a grade 1 acoustic neurinoma at the typical site of origin, near the junction of central and peripheral myelination.
Operative Techniques for Removing Acoustic Neurinomas Relative to the Size and Extension of the Tumor (Overview)
(Fig. 5.6)
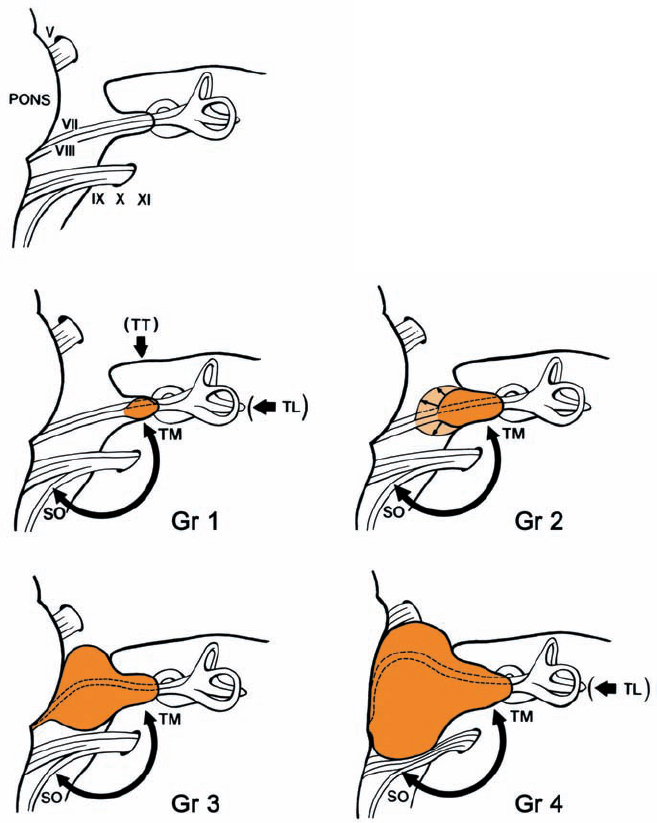
Fig. 5.6 The four tumor grades and the corresponding surgical approaches suggested. Grade 1: where hearing is preserved, either an extradural subtemporal approach or a suboccipital transmeatal approach is preferred. Only in cases in which hearing is absent can the translabyrinthine approach be recommended. Grade 2: the same approaches can be used, although the suboccipital approach, with its full visualization of the intracranial course of the nerves, is preferable. Grades 3 and 4: visualization of the brain stem and cranial nerves makes the suboccipital transmeatal approach the procedure of choice here.
Surgical Management of Acoustic Neurinomas
Small Acoustic Neurinomas (Grades 1 and 2)
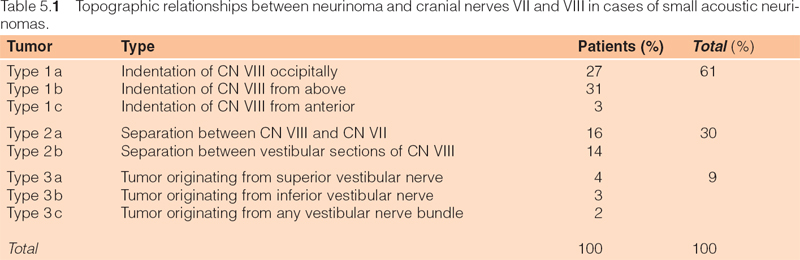
General Remarks (Figs. 5.7, 5.8; Table 5.1)
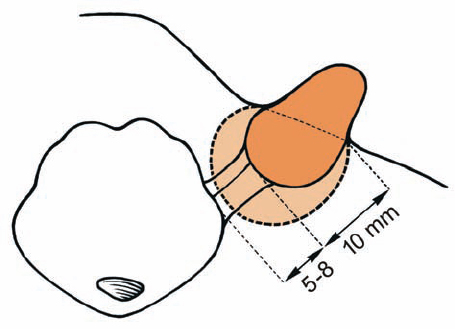
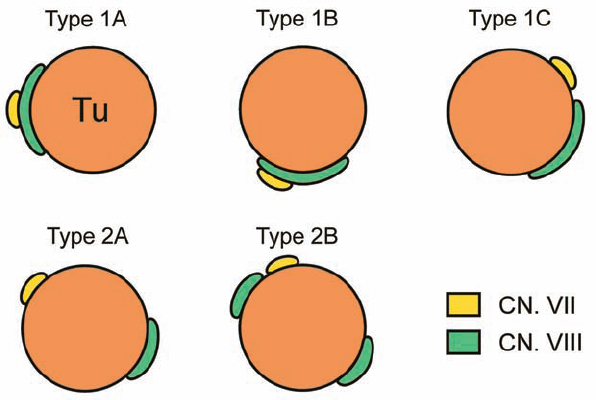
Fig. 5.7 Grade 2 tumors are divided into two subtypes, based on the extrameatal size of the tumor. This division has prognostic implications related to facial nerve and hearing outcomes. Grade 2 a tumors do not extend more than 10 mm into the cerebellopontine angle (CPA), measured from the lip of the porus acusticus, and grade 2 b tumors extend 11–18 mm into the CPA from the porus acusticus. This means that grade 2 a tumors provide more space between the tumor itself and the brain stem (5–8 mm).
Patterns and Types, Including Special Operative Techniques (Figs. 5.9–5.16)
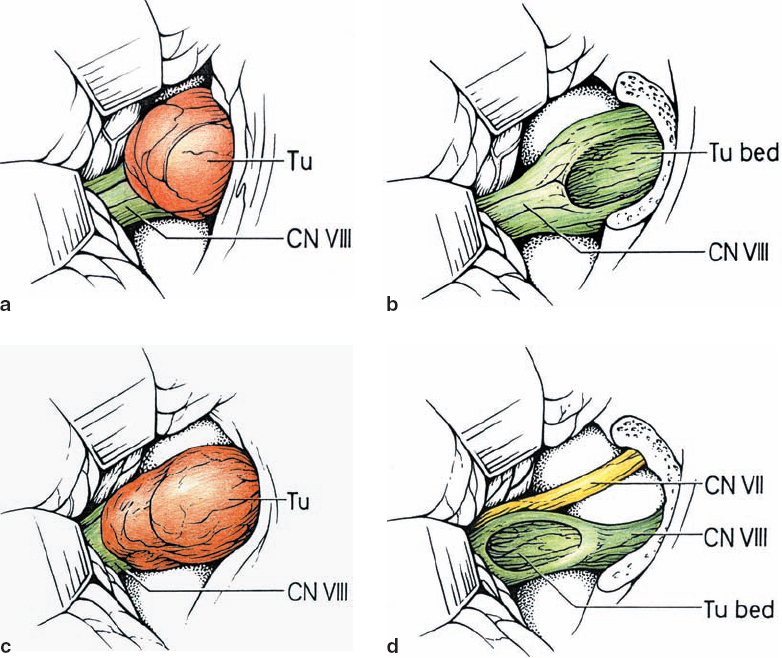
Fig. 5.9 a–d Type 1 a tumors indent the CN VIII complex from an occipital origin. Preresection and postresection illustrations are shown here.
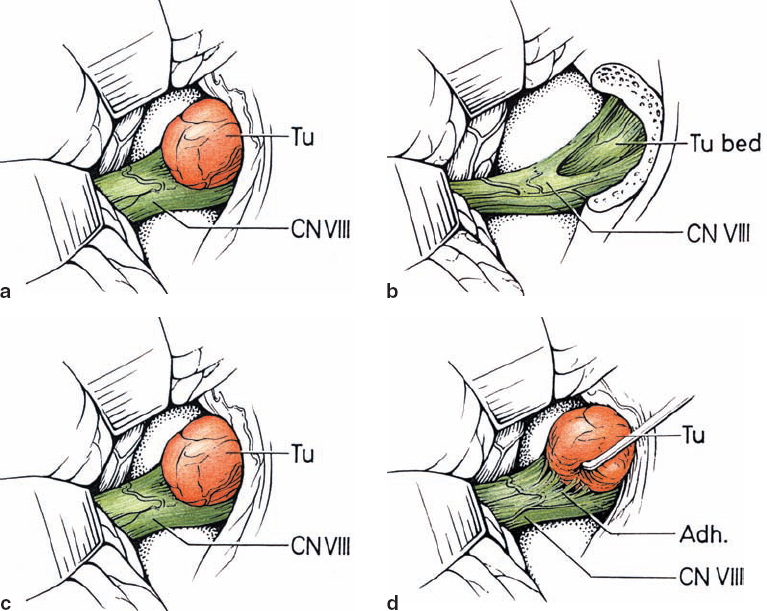
Fig. 5.10 a–d The lesion indents and displaces nerve fibers from above in type 1 b tumors. As shown in d, these tumors tend to be easily separable from the adherent nerve fibers.
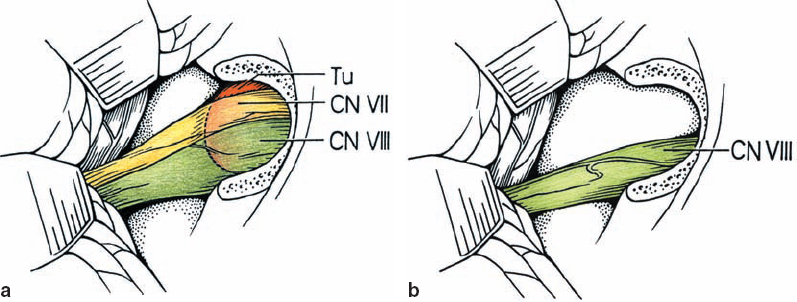
Fig. 5.11 a, b The least common lesions are type 1 c tumors, which displace the nerves from an anterior origin. This places the nerves between the surgeon and tumor, making for a much more difficult dissection.
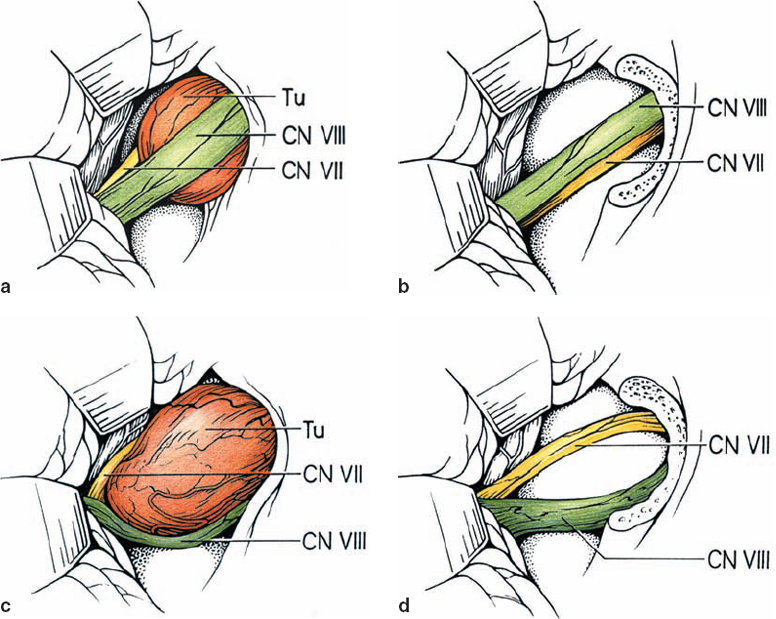
Fig. 5.12 a–d Type 2 a lesions open the natural plane between cranial nerves VII and VIII; CN VIII remains in a nicely compact bundle.
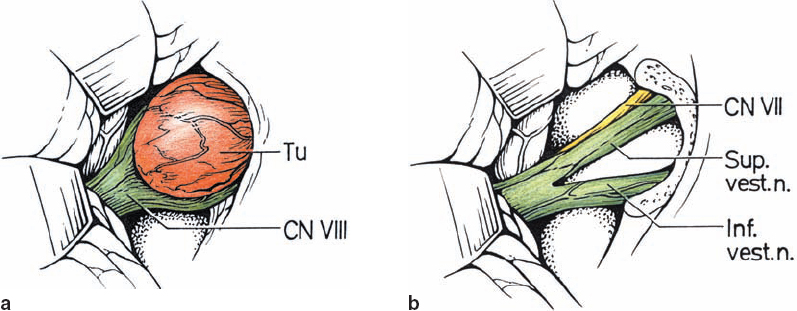
Fig. 5.13 a, b The vestibular nerve divisions are split by type 2 b tumors, as shown. The cochlear division tends to course adjacent to the inferior vestibular nerve.
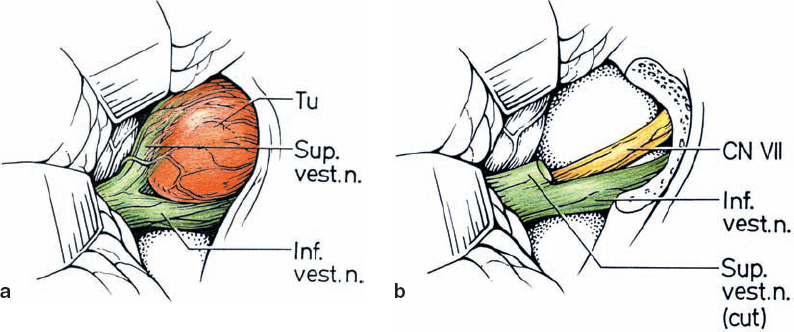
Fig. 5.14 a, b Type 3 a tumors are distinguished by a higher degree of vestibular nerve distortion, making it essentially impossible to dissect the tumor away and leave patent nerve fibers intact. As shown, type 3 a lesions arise from the superior vestibular nerve.
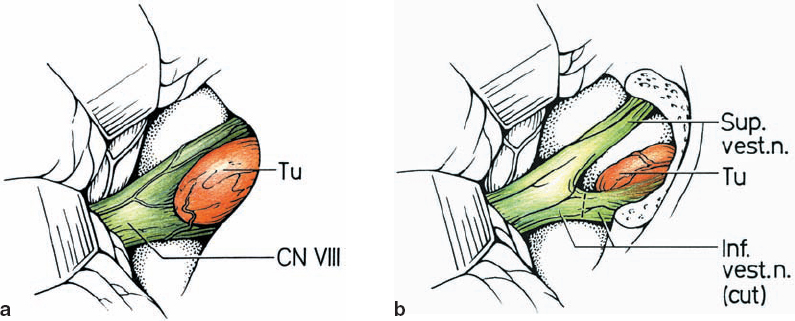
Fig. 5.15 a, b The inferior vestibular nerve is the origin of type 3 b lesions.
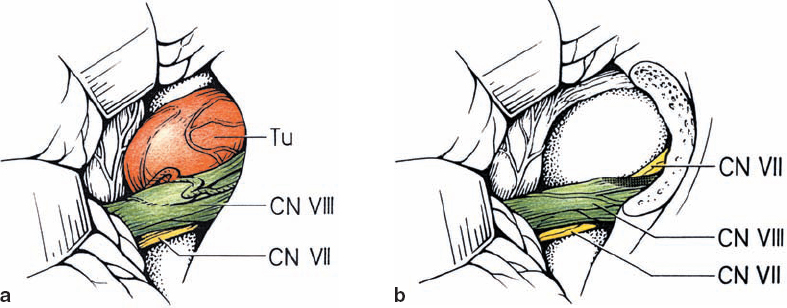
Fig. 5.16 a, b Type 3 c lesions are overall the least common topographic configuration. The nerve of origin is not distinguishable.

Fig. 5.17 a Intracanalicular right-sided acoustic neurinoma grade 1. An axial postcontrast CT section at the level of the internal acoustic canal. Note the globular contour of the small neurinoma, which does not extend to the fundus of the meatus.
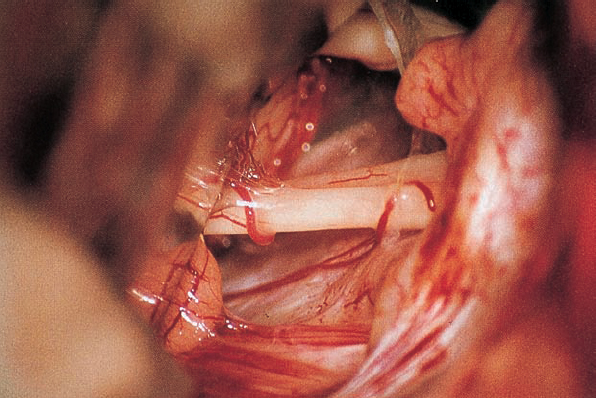
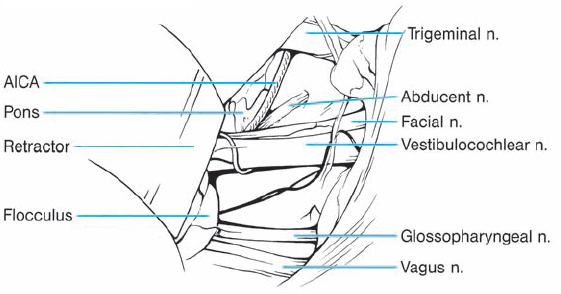
Fig. 5.17 b The right cerebellopontine angle is exposed during a standard suboccipital retromastoid approach. The upper lip of the internal acoustic porus is overlapped by a bony protrusion. No tumor is seen protruding out of the internal meatus.
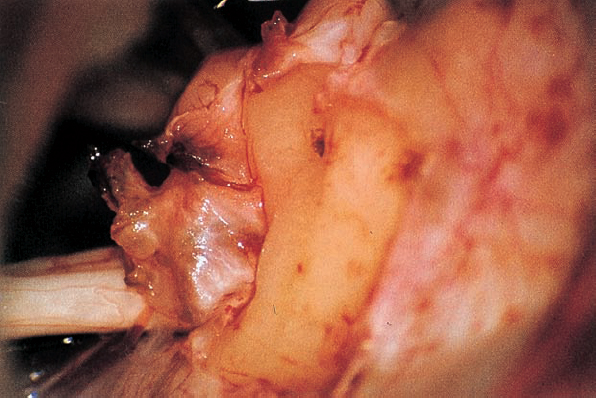
Fig. 5.17 c The dura overlying the posterior wall of the internal acoustic meatus is coagulated and incised with a blade 11 knife; the small dural flap is separated from the bone and pushed to the posterior lip of the internal acoustic porus.
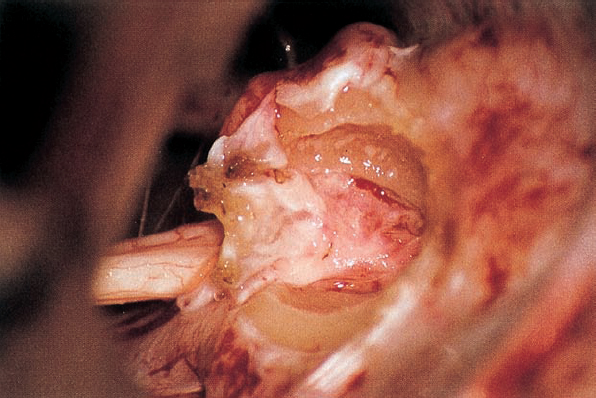
Fig. 5.17 d The posterior wall of the internal acoustic meatus is removed with a high-speed Midas-Rex diamond drill. The dura of the internal meatus, resembling a cul-desac, is exposed.
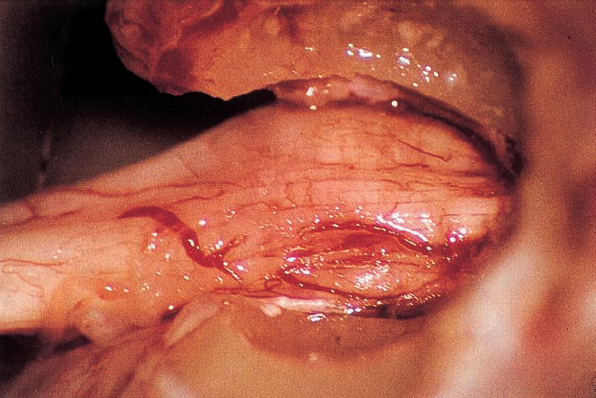
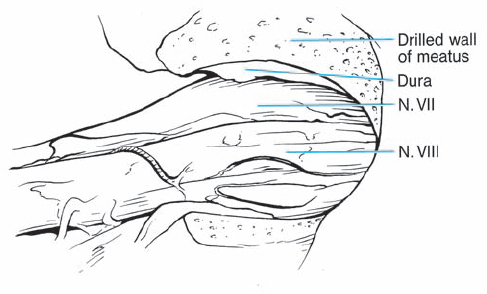
Fig. 5.17 e After resection of the dura, the intrameatal nervous structures are exposed. Cranial nerves VIII and VII are displaced posteriorly by the neurinoma, which is located in front of the nerves.
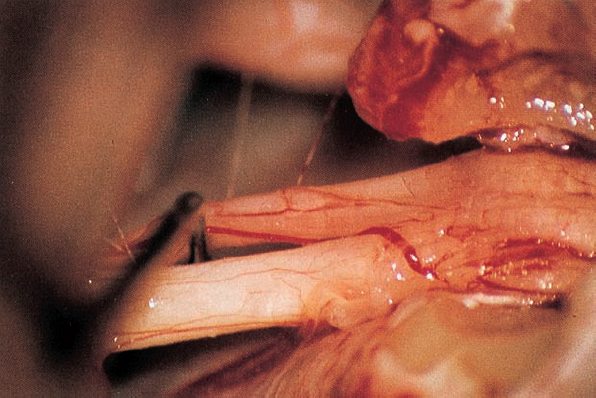
Fig. 5.17 f A blunt hook separates nerves VIII and VII.
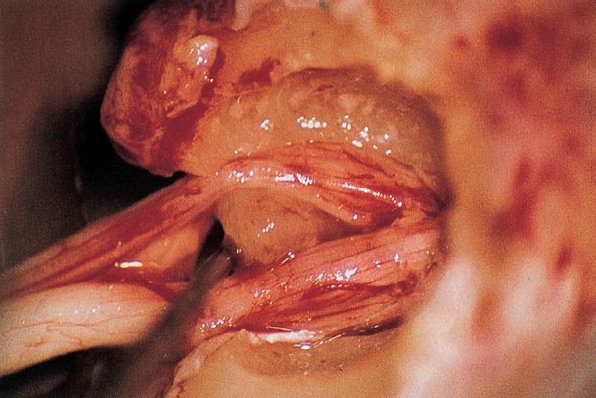
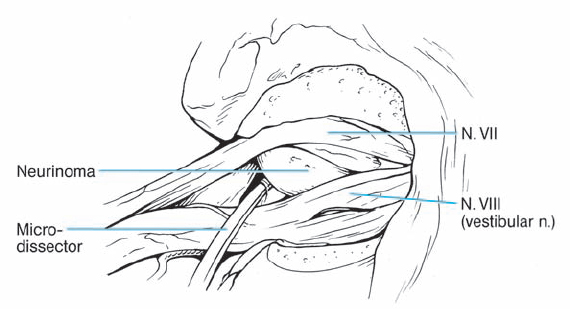
Fig. 5.17 g By spreading the bundles of the seventh and eighth nerve, the nodule of the neurinoma is brought into view.
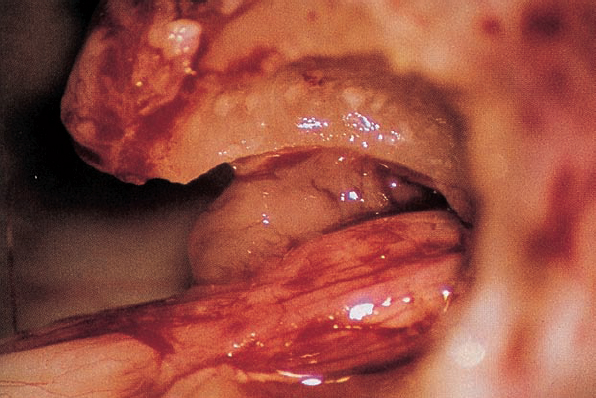
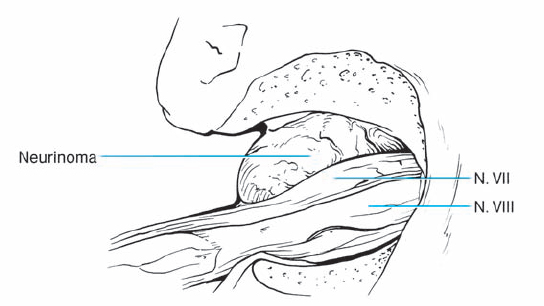
Fig. 5.17 h Downward displacement of the facial nerve exposes the tumor lying in front.
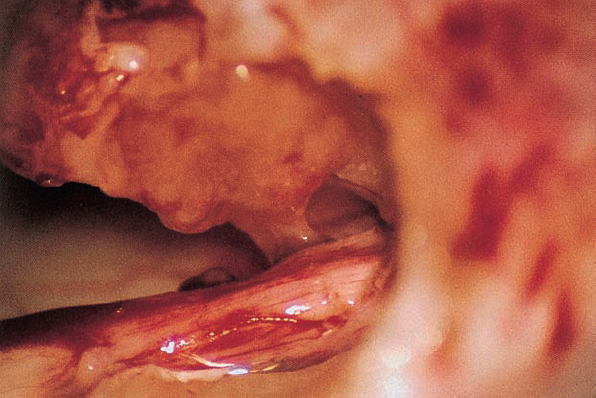
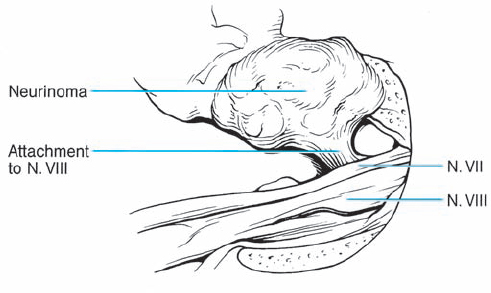
Fig. 5.17 i The attachment of the acoustic neurinoma can be visualized.
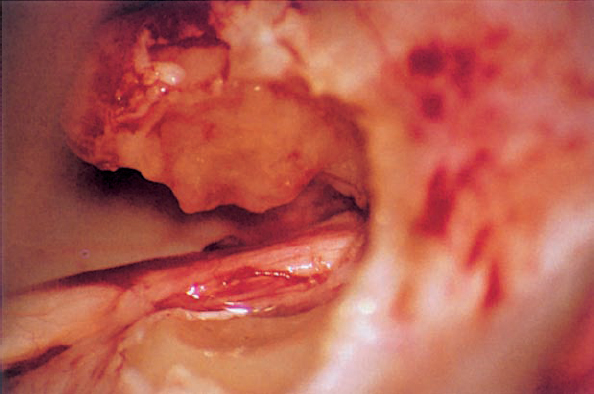
Fig. 5.17 j The tumor is separated from the eighth nerve; the connection between the neurinoma and the nerve has been cut.
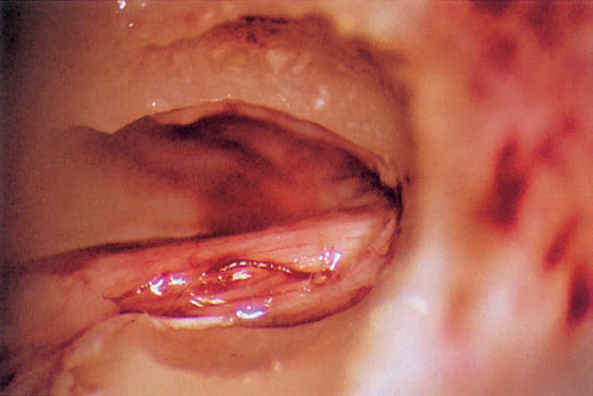
Fig. 5.17 k The seventh and eighth nerve bundle can be seen within the enlarged internal acoustic meatus.
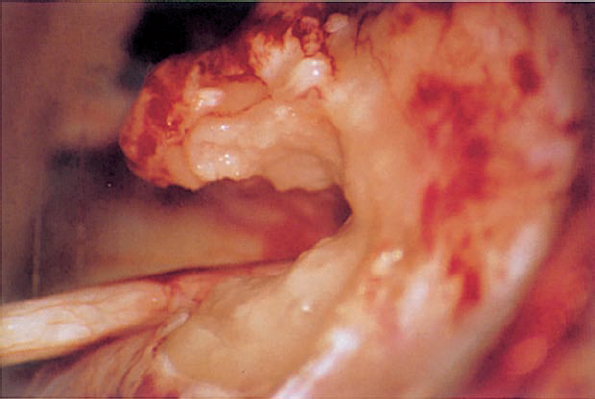
Fig. 5.17 l The margins of the posterior meatal wall are sealed with fibrin glue and small plates of Gelfoam (or pieces of muscle). The preoperative hearing is preserved.
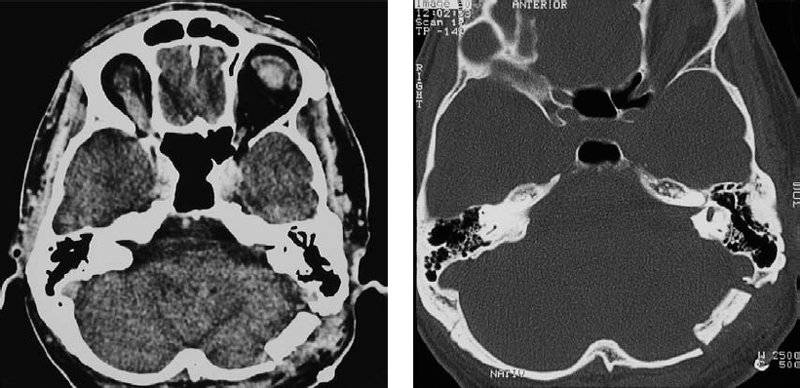
Fig. 5.17 m, n Postoperative CT scans with contrast enhancement (m) and with a high-resolution bone window (n), demonstrating the size of the approach and the piece of bone removed at the entrance of the inner meatus.
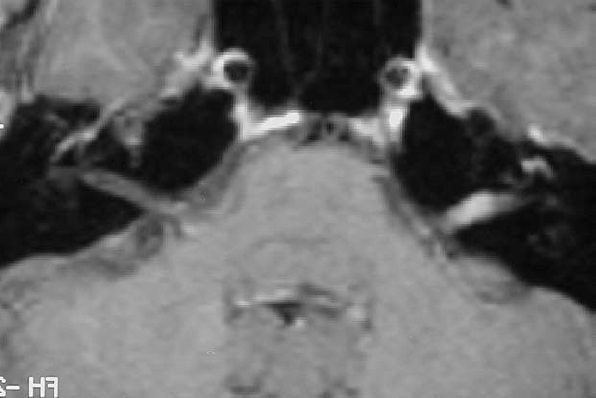
Fig. 5.18 a Axial contrast-enhanced MRI, demonstrating a grade 1 acoustic neurinoma in a 33-year-old woman.
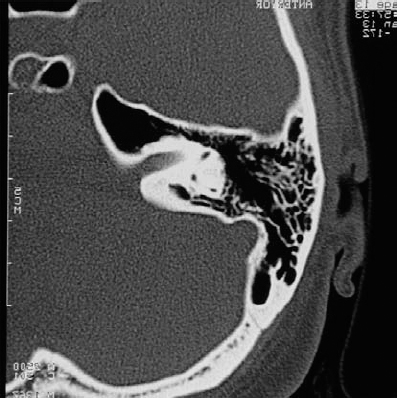
Fig. 5.18 b A high-resolution bone window CT scan demonstrates relative lack of erosion of the internal auditory canal (IAC). A significant air cell complex is located around the IAC. It appears that exposure to the fundus is possible without fenestration of the labyrinth.
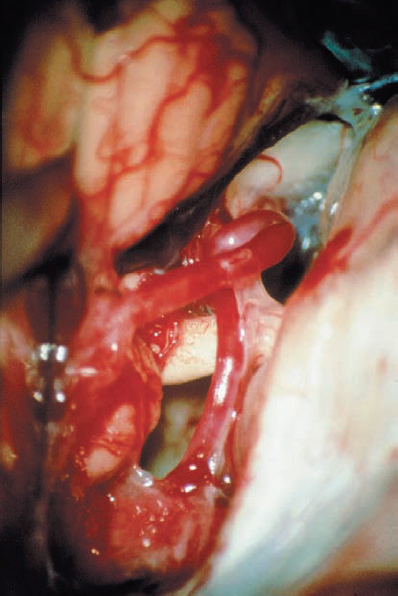
Fig. 5.18 c Initial view of the right cerebellopontine angle (CPA) via a retrosigmoid approach.
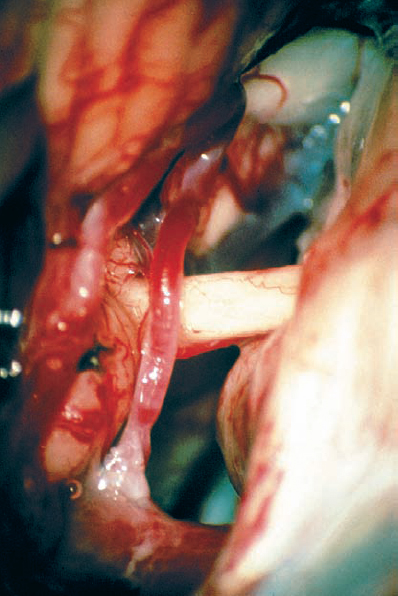
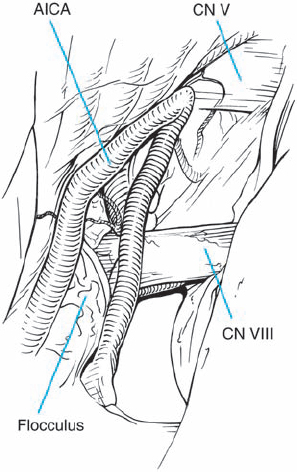
Fig. 5.18 d Arachnoid dissection frees the anterior inferior cerebellar artery (AICA), allowing posterior mobilization, away from the porus acusticus.
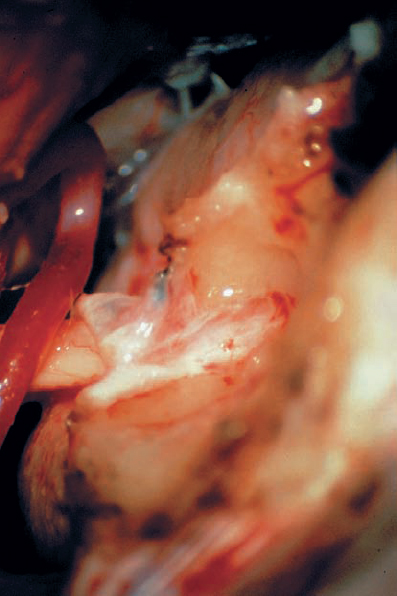
Fig. 5.18 e A dural flap has been raised and reflected medial. The bone of the back wall of the IAC has been removed, exposing the dura lining the IAC.
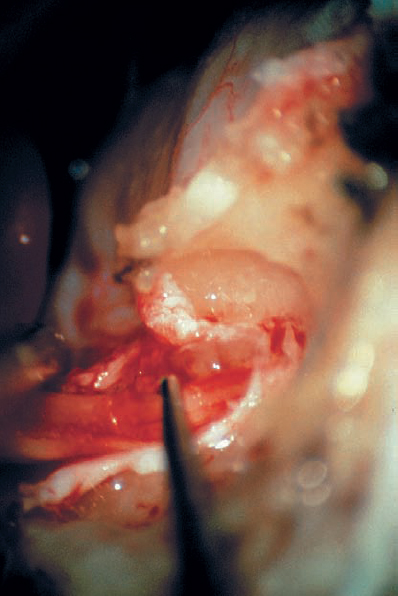
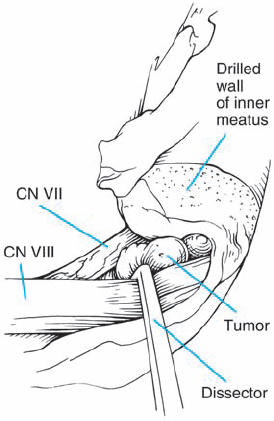
Fig. 5.18 f The dura is opened. A short nerve hook gently retracts the superior vestibular nerve inferiorly, bringing the small tumor into view.
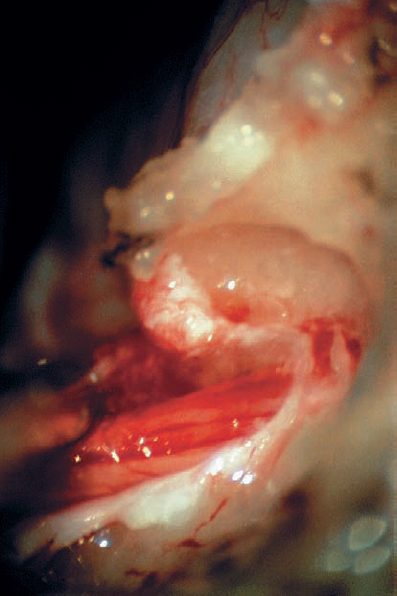
Fig. 5.18 g The tumor originates from the superior vestibular nerve. The facial nerve is located anterior and medial to the tumor.
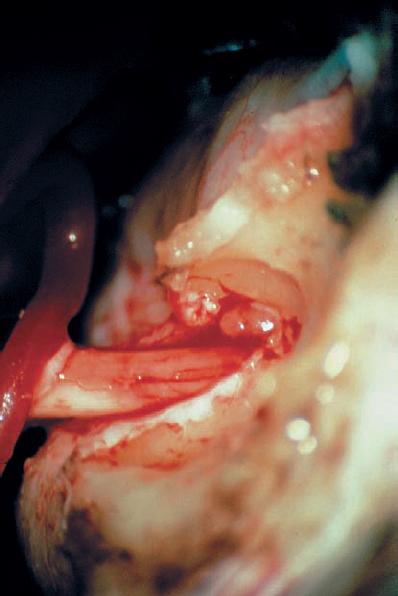
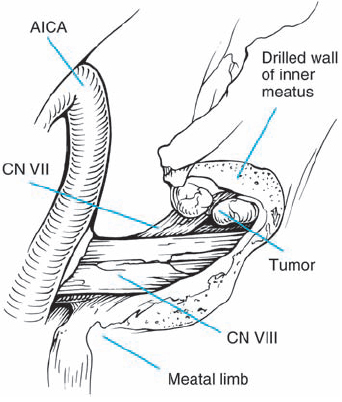
Fig. 5.18 h The tumor is removed in a piecemeal fashion.
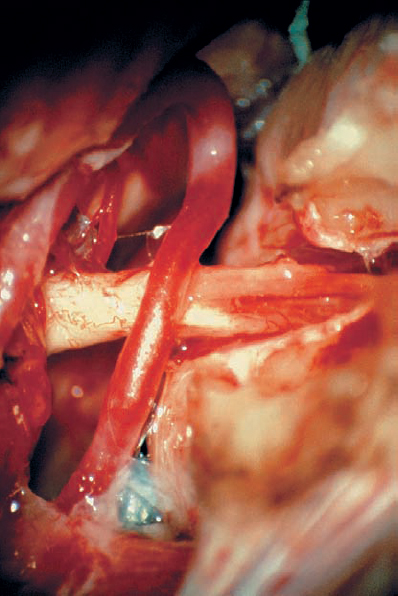
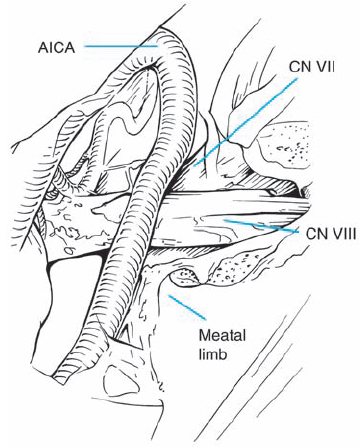
Fig. 5.18 i The tumor is completely removed, while preserving all cranial nerve structures.
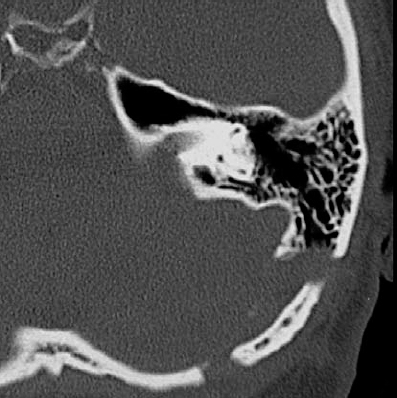
Fig. 5.18 j A postoperative bone window CT demonstrates removal of the posterior wall of the IAC and reconstruction of the calvaria.
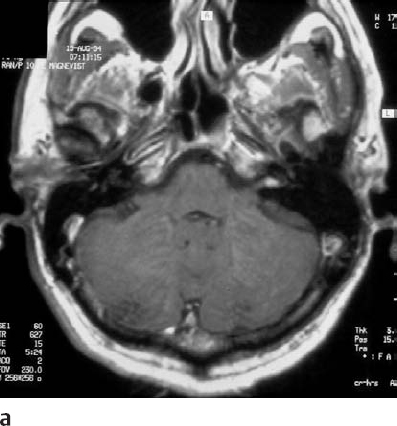
Fig. 5.19 a An axial MRI with contrast reveals an intracanalicular grade 1 acoustic neurinoma. In such cases, when hearing is good, the patient has a high chance of hearing preservation with removal of the tumor. The recurrence rate is generally quite low for such tumors, and surgery was therefore offered to this patient as a good treatment option.
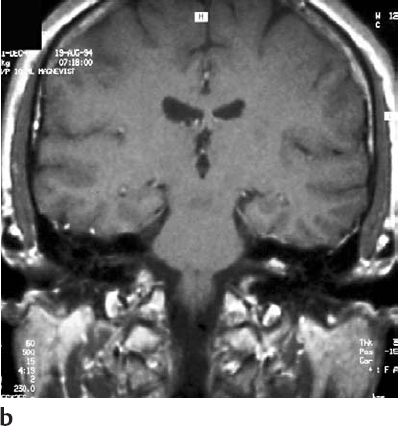
Fig. 5.19 b Coronal contrast-enhanced MRI at the level of the internal auditory canals, demonstrating the tumor.
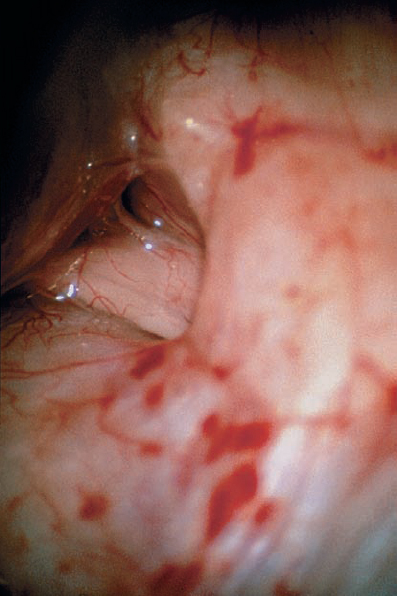
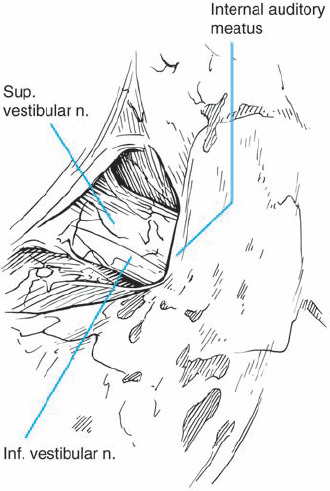
Fig. 5.19 c Via a retrosigmoid approach, the typical view is obtained, with no tumor visible at the porus acusticus. To plan removal of the posterior wall of the IAC, the location of the endolymphatic sac is determined, which marks the lateral limit of the dural incision of the posterior petrosal surface.
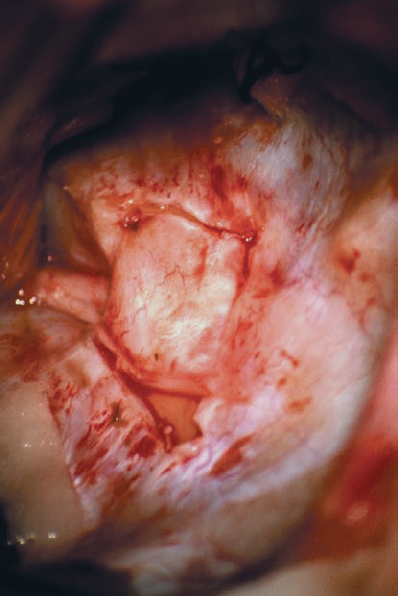
Fig. 5.19 d The dura is incised in a semilunar shape, with the lateral extent approaching the bony operculum. The operculum is a bony ledge that overhangs the vestibular aqueduct entrance into the endolymphatic sac. Respecting this lateral limit will preserve the integrity of the endolymphatic sac and avoid potential hearing loss due to loss of endolymph.
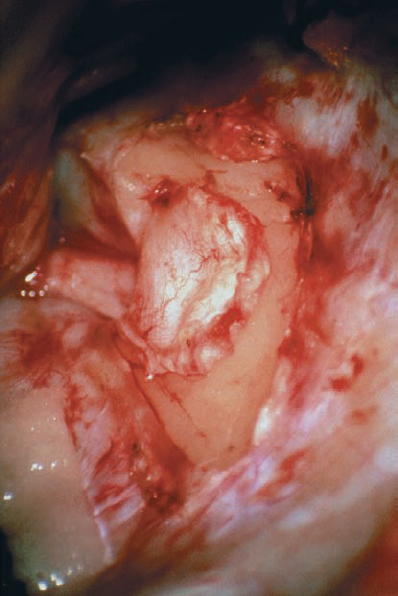
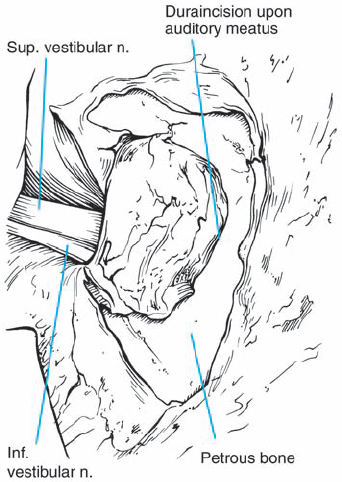
Fig. 5.19 e The dura is reflected using a sharp elevator in a medial direction, so that it can be used as a cover over the normal nerve structures. This provides some degree of protection, by placing an anatomical barrier between the nerve structures and the drill tip.
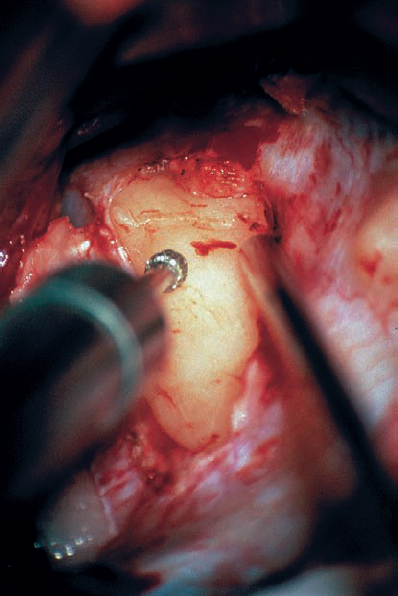
Fig. 5.19 f Drilling is performed using a small diamond tip, typically no more than 2.5 mm in diameter. Drilling is carried out with continuous irrigation flowing over the area, to dissipate the heat produced by the diamond burr.
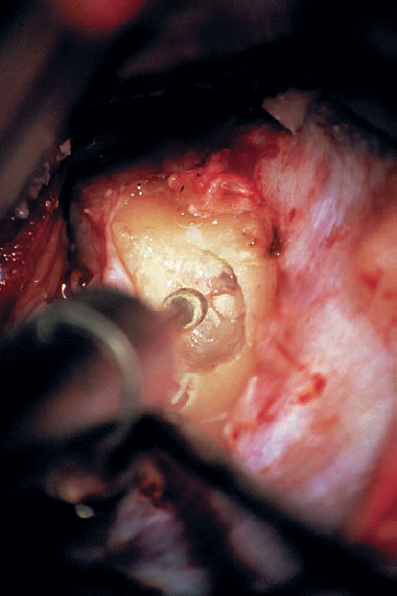
Fig. 5.19 g Bone is removed in a medial to lateral direction, beginning at the porus acusticus. The superior and inferior margins of the canal are defined in order to maximize exposure. Before surgery, the lateral extent of drilling is determined by careful consideration of the imaging studies. Particular attention is paid to the relationship of the vestibule, common crus, and posterior semicircular canal to the internal auditory canal.
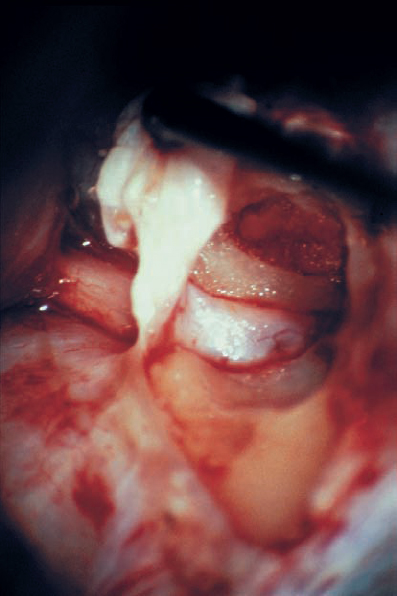
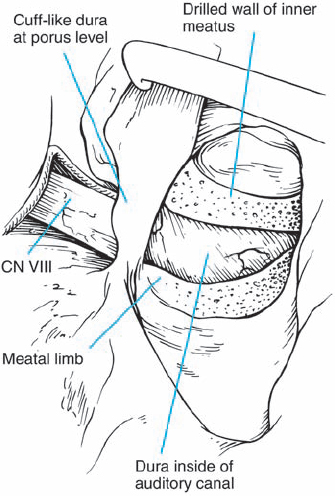
Fig. 5.19 h Bone removal is completed. Ideally, the dura lining the canal should be left intact during this process, as is shown here.
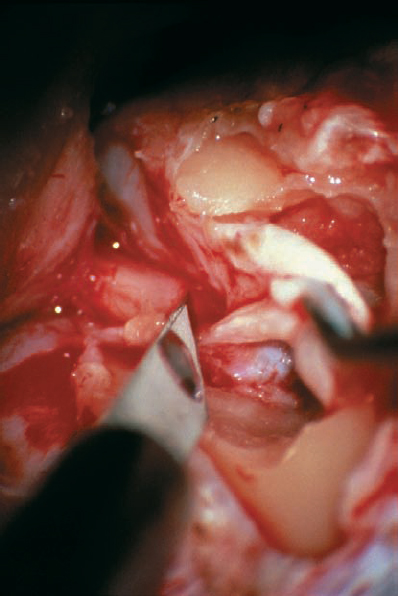
Fig. 5.19 i The dura is then carefully incised over the canal to fully expose the contents of the IAC. The dural flap was reflected superiorly in this case.
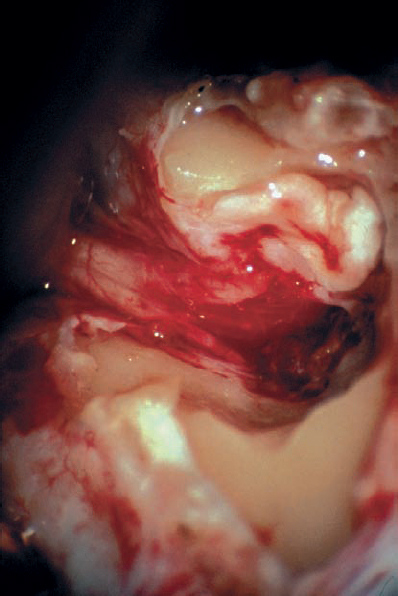
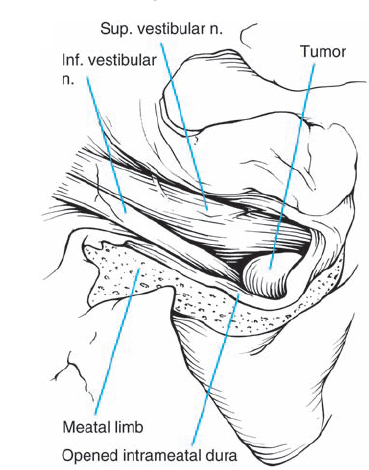
Fig. 5.19 j The nerves in the IAC and the lateral aspect of the tumor in are exposed in this view. The tumor has been partially removed at this stage. In such cases, it may be difficult to remove the posterior IAC wall to achieve full exposure of the fundus region, necessitating the use of an endoscope or mirror to provide good visualization of the area.
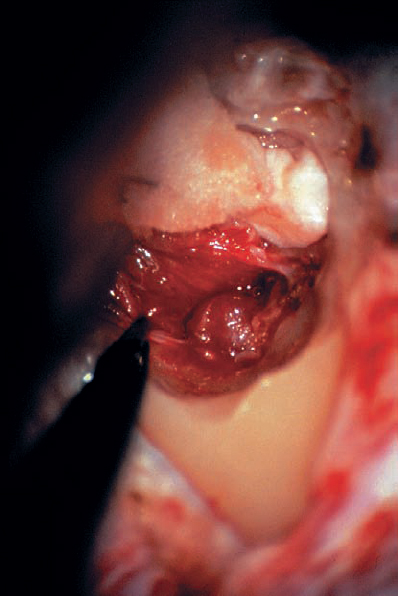
Fig. 5.19 k Residual parts of the tumor can be totally removed.
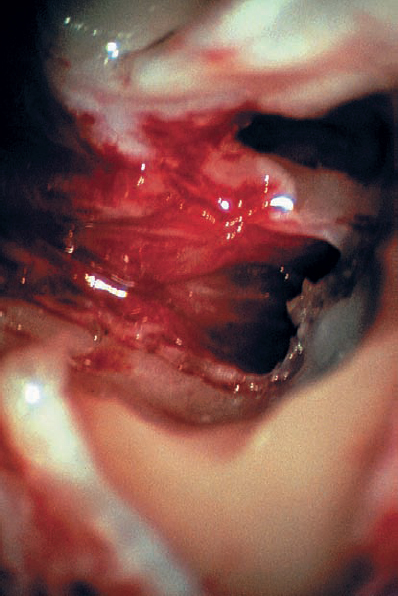
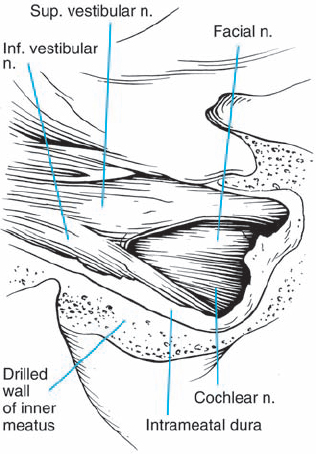
Fig. 5.19 l The final view, with the tumor removed and the stump of the vestibular nerves cut at the lateral end of the canal. Hearing was preserved in this patient.
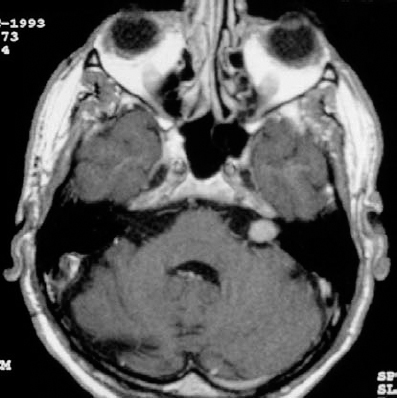
Fig. 5.20 a A contrast-enhanced axial MRI of a grade 2 a tumor on the right side.
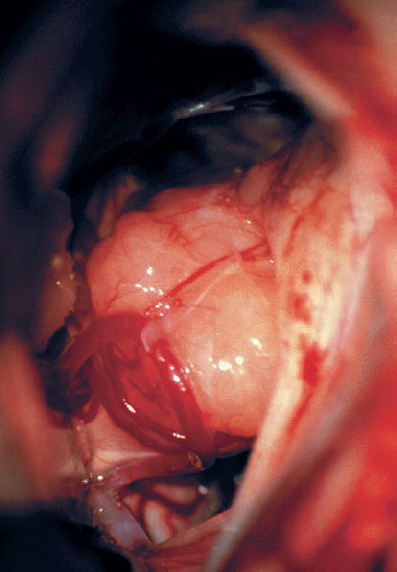
Fig. 5.20 b The intraoperative view of the right CPA shows the tumor extending in an extrameatal direction.
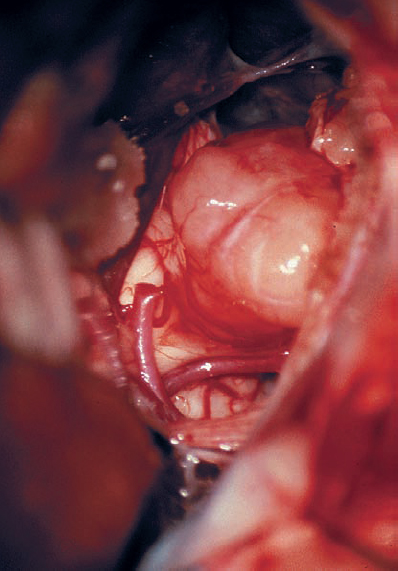
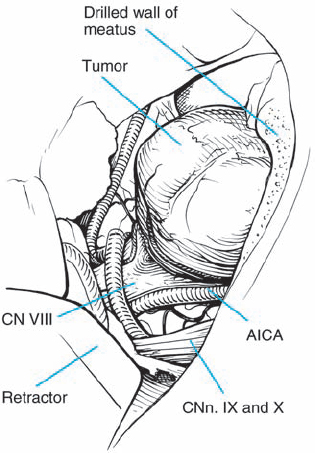
Fig. 5.20 c Limited removal of the posterior wall of the IAC has been carried out to expose more tumor in the canal.
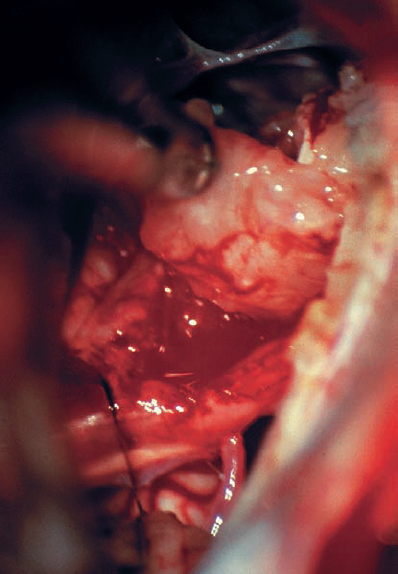
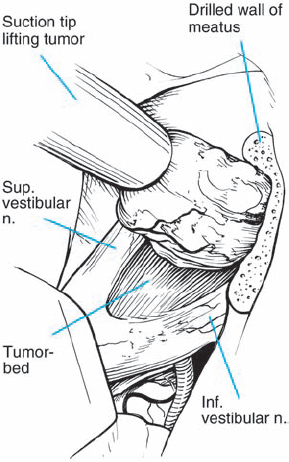
Fig. 5.20 d The tumor is gently dissected away, and is seen here being retracted superiorly with suction. The mass is seen deforming the eighth nerve from the occipital direction, showing that this is a type 1 a topographic configuration.
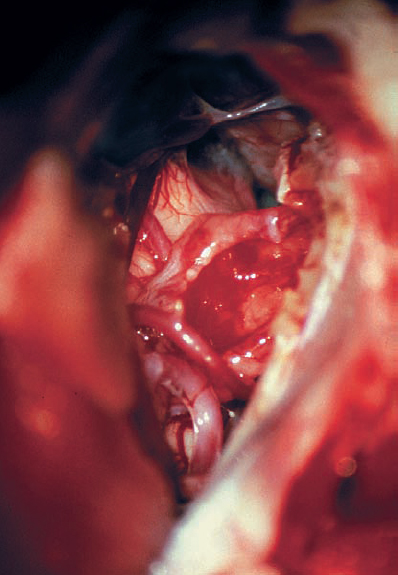
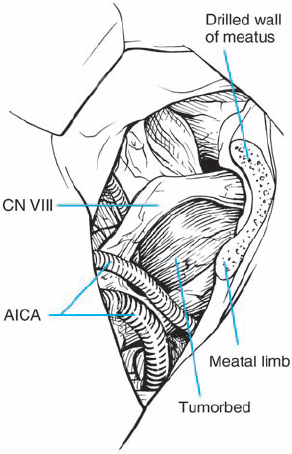
Fig. 5.20 e After tumor removal, the distortion caused by the mass is clearly appreciated.
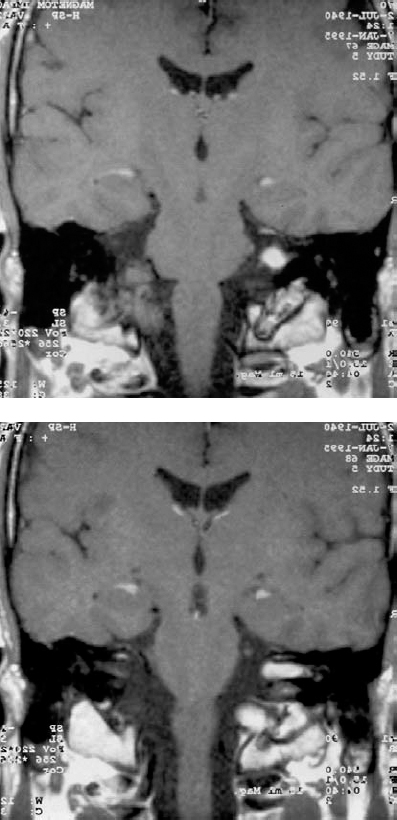
Fig. 5.21 a, bCoronal contrast-enhanced MRI views of a grade 2 a mass on the right side.
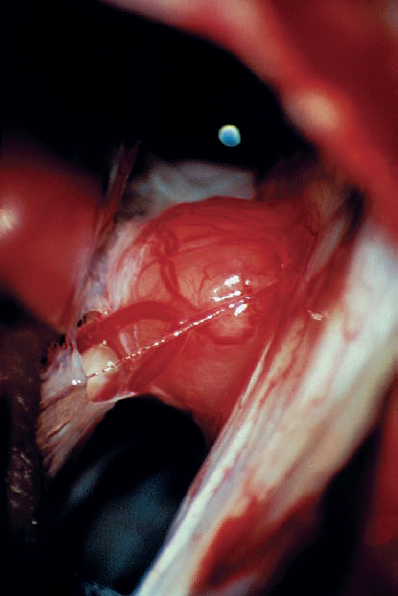
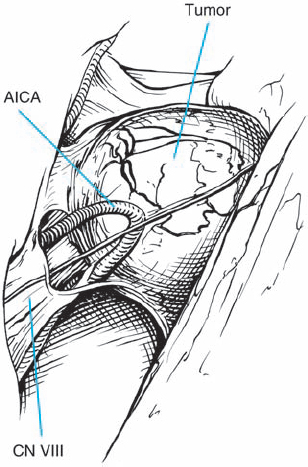
Fig. 5.21 c The intraoperative view via a right retrosigmoid approach demonstrates the tumor after arachnoid dissection.
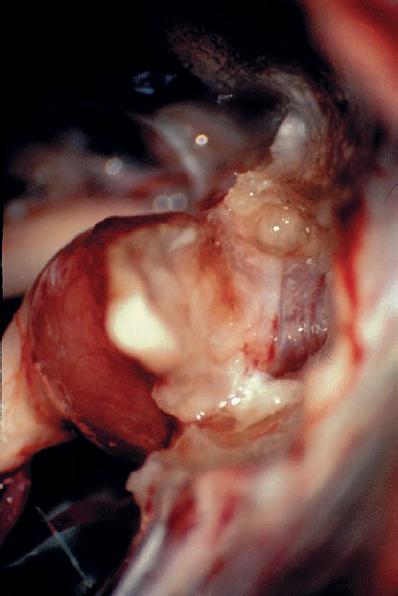
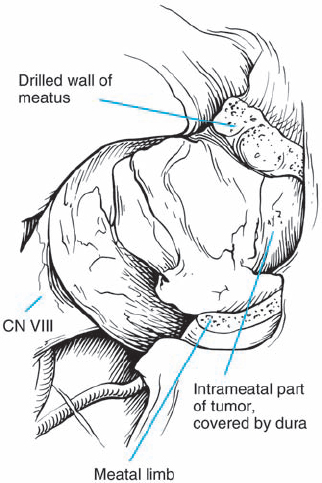
Fig. 5.21 d The petrosal dural flap has been reflected, and the necessary length of the posterior IAC wall has been drilled away.
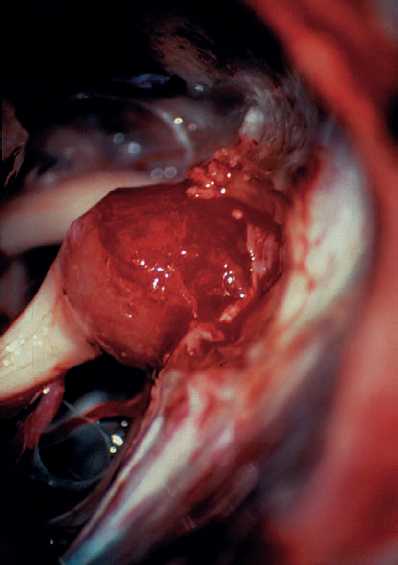
Fig. 5.21 e The tumor is exposed and debulked before dissection from the nerves. The occipital origin, with displacement of the eighth nerve fibers, can begin to be discerned, showing that this is a type 1 a configuration.
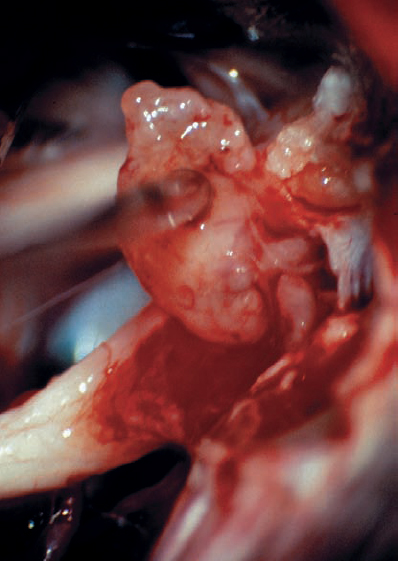
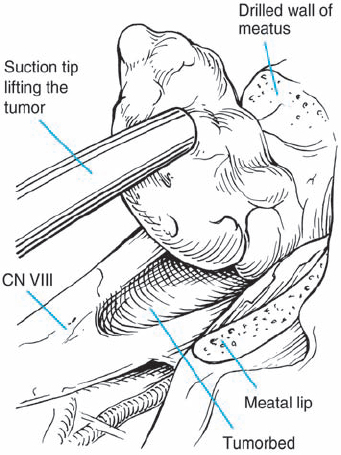
Fig. 5.21 f With gentle dissection of the tumor–nerve interface, the mass is elevated out of the canal.
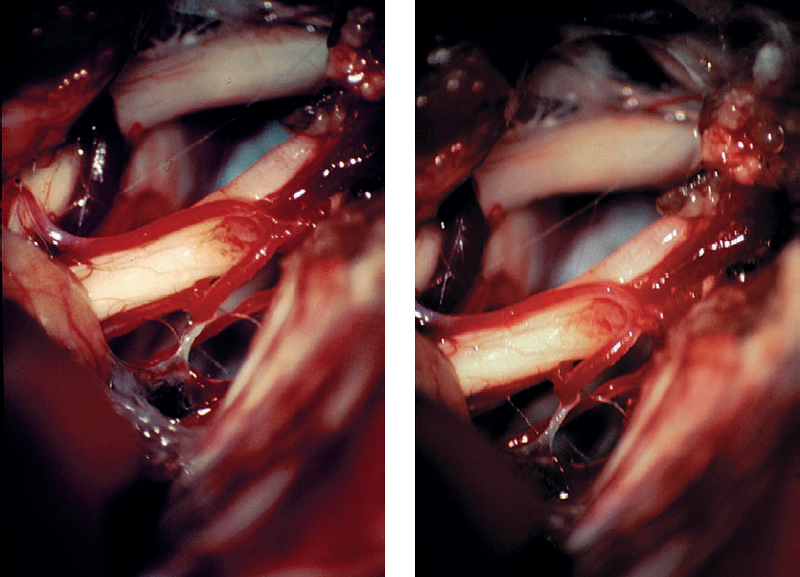
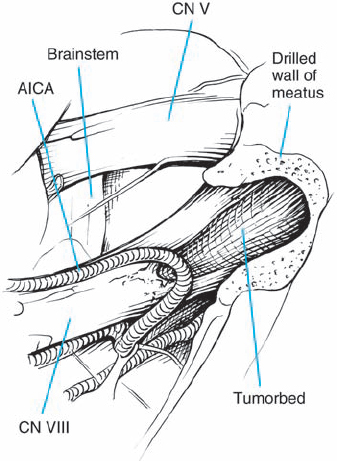
Fig. 5.21 g, h Slightly different microscopic views of the CPA after tumor removal, showing preservation of the vascular structures and the eighth nerve.
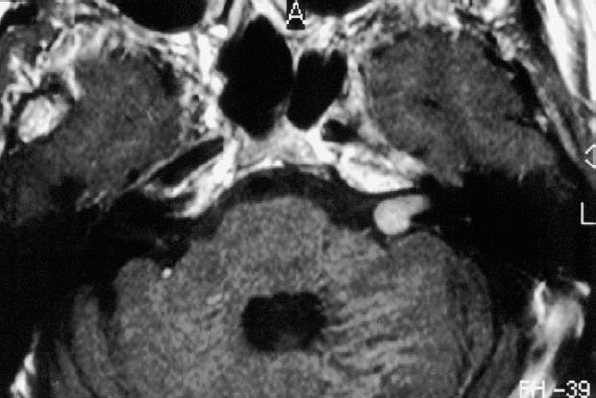
Fig. 5.22 a A preoperative contrast-enhanced axial MRI view of a right-sided grade 2 a acoustic neurinoma, detected in a 55-yearold man with good hearing.
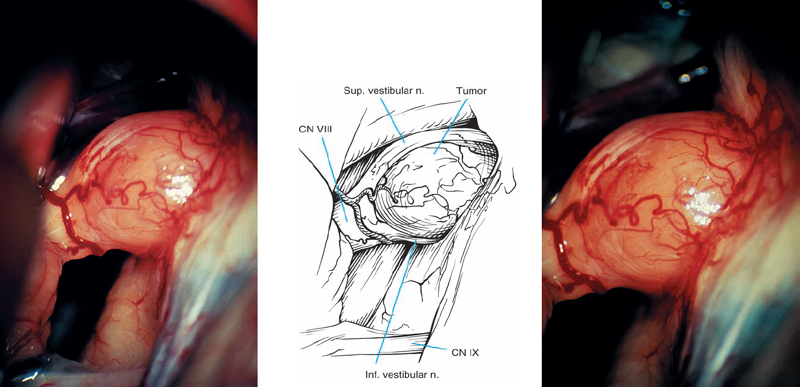
Fig. 5.22 b, c Intraoperative views at different magnifications demonstrate the tumor. The eighth nerve fibers can be discerned coursing over the top and bottom of the mass, suggesting type 1 a topography.
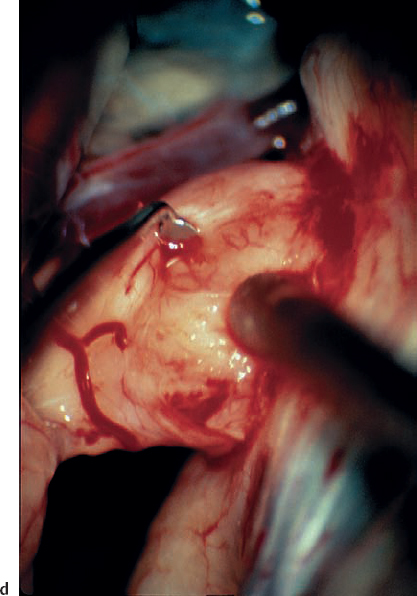
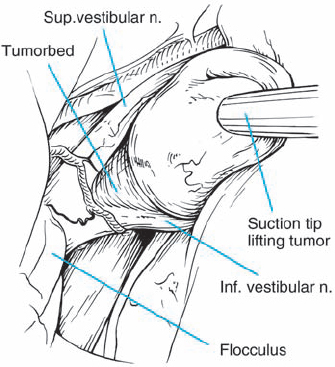
Fig. 5.22 d A plane of separation is developed between the tumor capsule and the nerve fibers with a fine, sharp-edged micro-dissector, while applying gentle traction with suction.
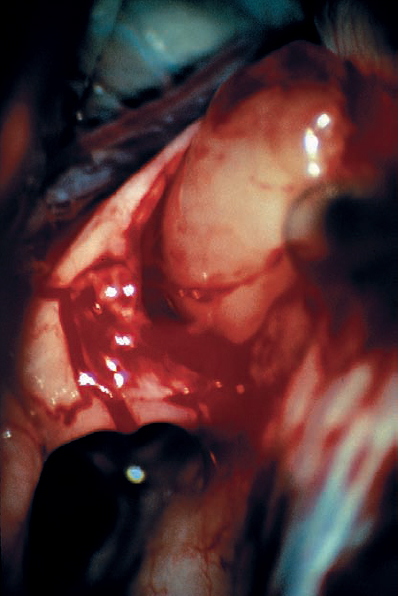
Fig. 5.22 e With the dissection plane developed, the posterior pole of the tumor is mobilized, and it is seen here separating nicely from the eighth nerve fibers.
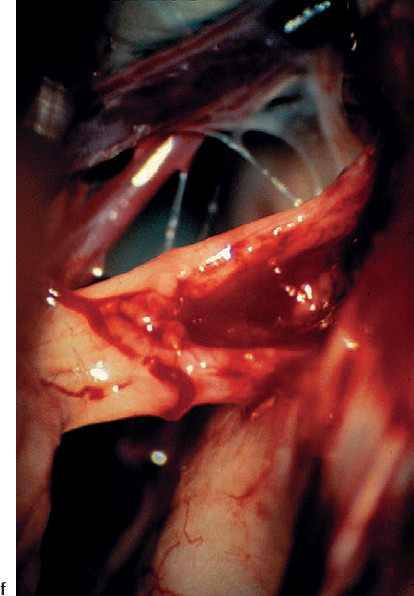
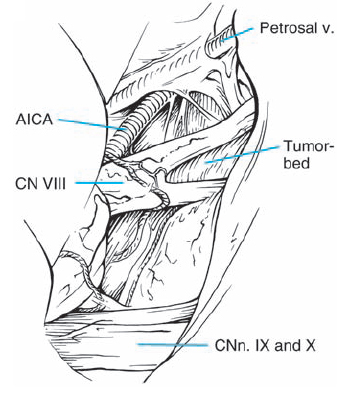
Fig. 5.22 f A magnified view of the eighth nerve after tumor removal shows the indentation of the eighth nerve caused by the type 1 a tumor.
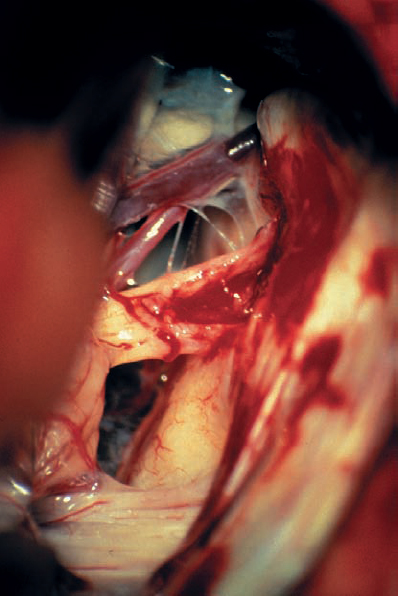
Fig. 5.22 g A panoramic view of the CPA after tumor removal shows the anatomic relationships between the adjacent cranial nerves and the brain stem in this case.
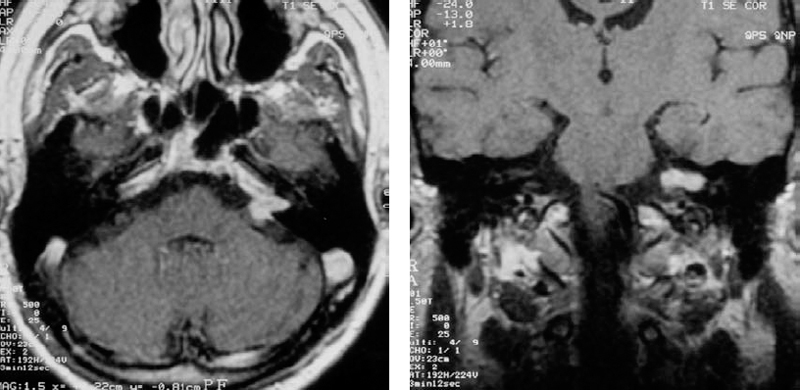
Fig. 5.23 a, b Coronal (a) and axial (b) contrast-enhanced MRI views in this patient demonstrate the cause of canal erosion, a grade 2 a acoustic neurinoma. Note the lack of bony erosion at the porus acusticus. A constriction of this type can make removal of bone in this area more difficult.
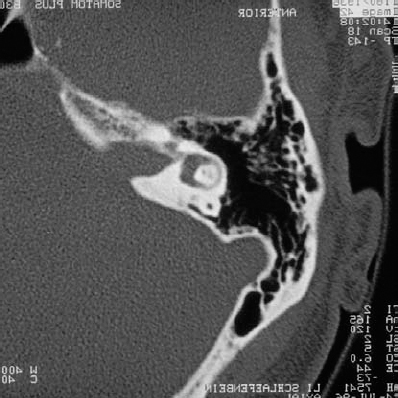
Fig. 5.23 c The axial bone window CT demonstrates an eroded IAC in this 60-yearold woman with symptoms of tinnitus.
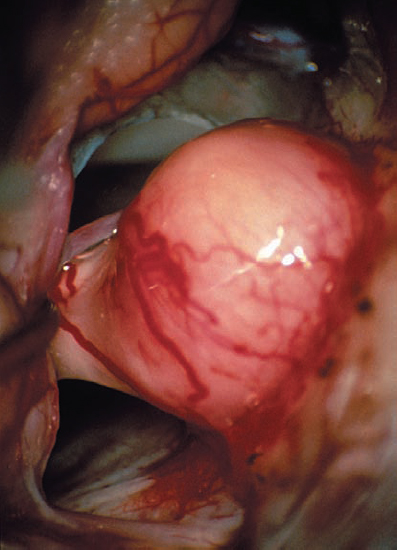
Fig. 5.23 d A view via the retrosigmoid approach of the CPA and tumor extending from the porus acusticus.
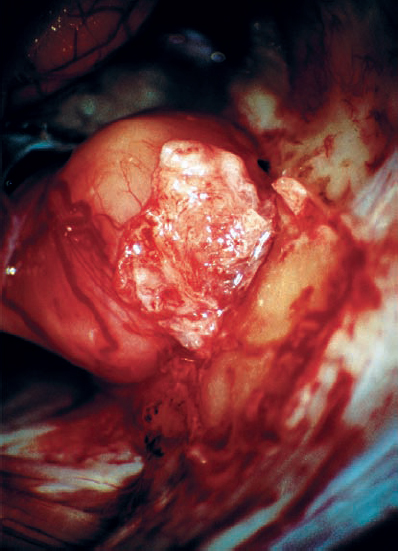
Fig. 5.23 e The dural flap is elevated before removal of the posterior wall of the IAC.
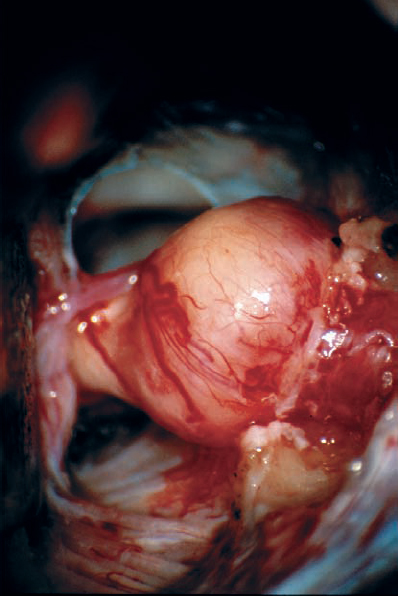
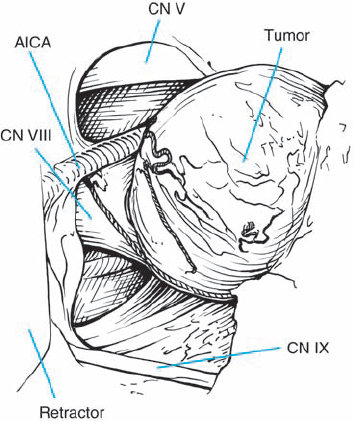
Fig. 5.23 f After bone removal, the tumor is exposed into the IAC, near the fundus.
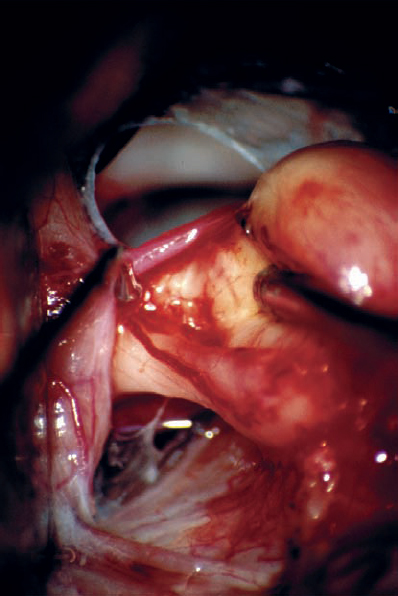
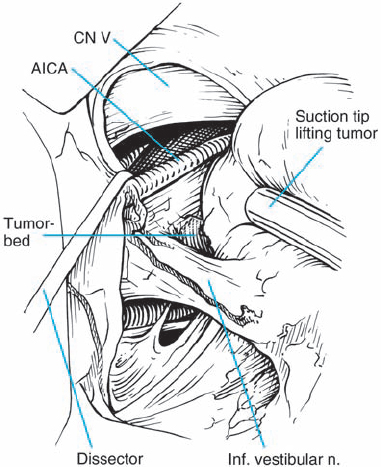
Fig. 5.23 g The tumor is gently elevated, and the nerve – tumor capsule interface is dissected. The nerve fiber bundles are seen to be distorted and deflected anterior and medial, suggesting type 1 a topography.
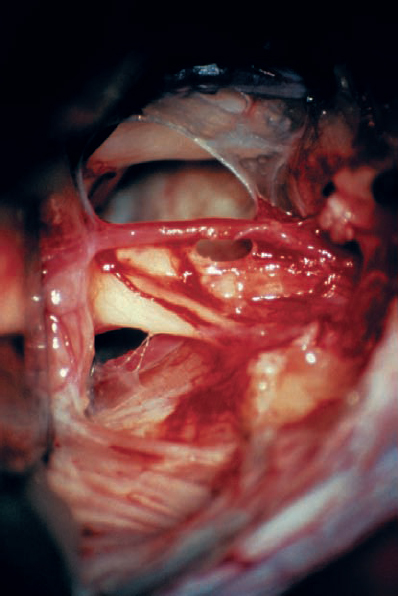
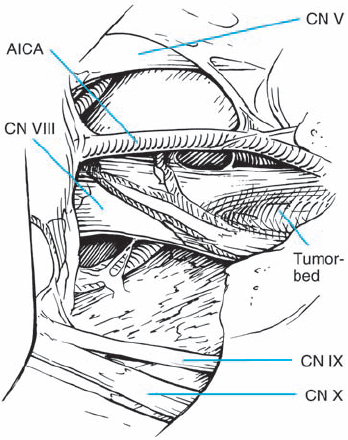
Fig. 5.23 h The tumor has been totally removed. Importantly, the AICA loop extending into the IAC and its branches have been preserved. A branch supplying the cochlear division can be seen coursing over the inferior and lateral aspect of the nerve as it enters the IAC. This type of vascular preservation is essential if hearing is to be preserved.
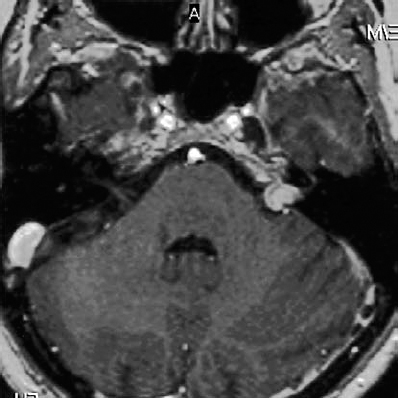
Fig. 5.24 a An axial MRI image with contrast enhancement shows a grade 2 a lesion.
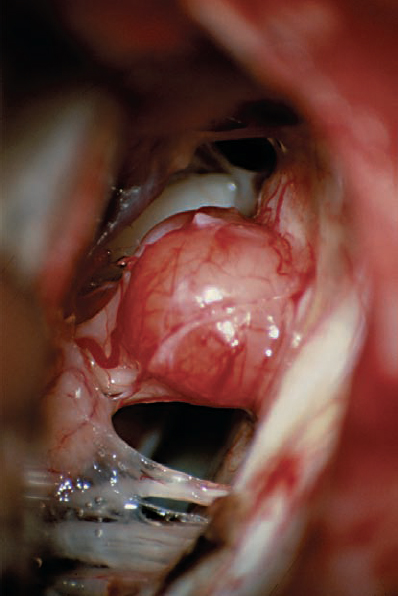
Fig. 5.24 b Intraoperative view of the CPA. The relationships of the adjacent cranial nerves are clearly demonstrated.
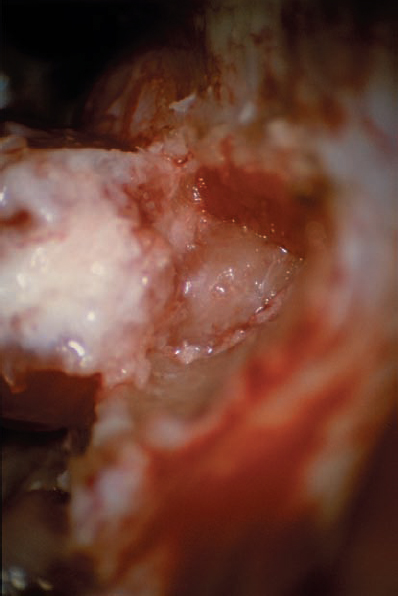
Fig. 5.24 c A dural flap is raised, and the posterior wall of the IAC is removed.
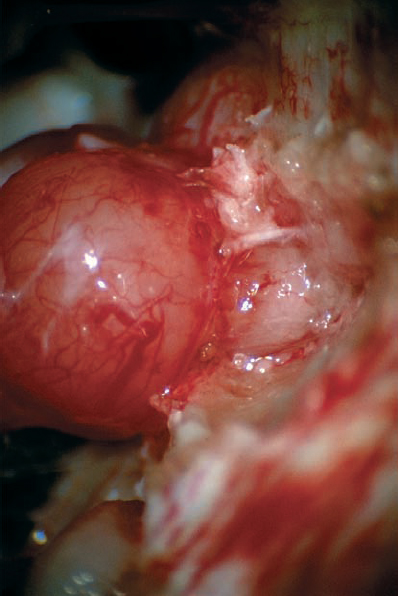
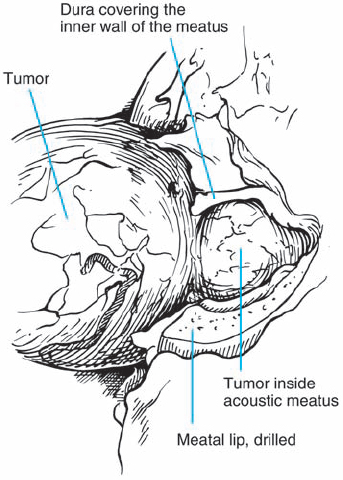
Fig. 5.24 d With the dura of the IAC opened, the intracanalicular portion of the tumor is exposed and can be debulked.
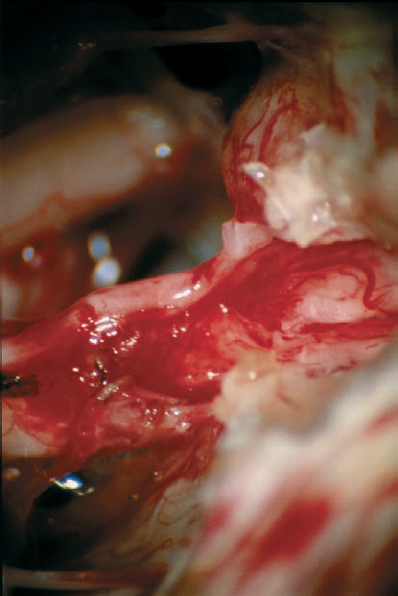
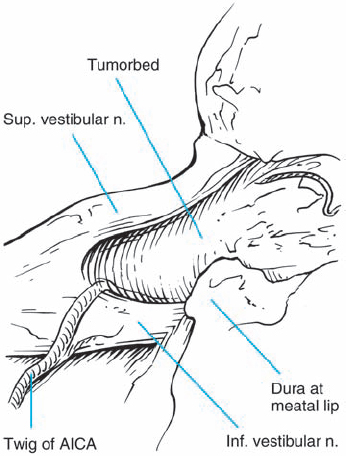
Fig. 5.24 e After total tumor removal, the type 1 a topography can be appreciated. Note also the preserved auditory artery.
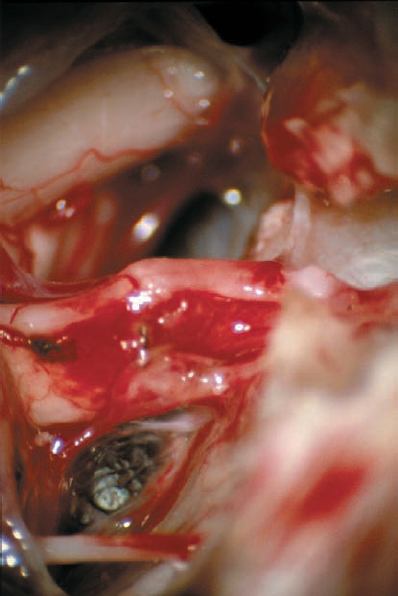
Fig. 5.24 f A slightly different view after tumor removal, compared with e.
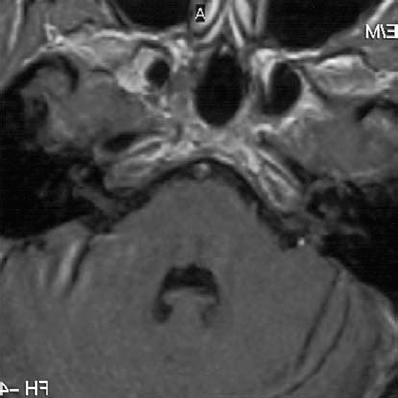
Fig. 5.24 g The postoperative axial MRI image shows total removal of tumor.
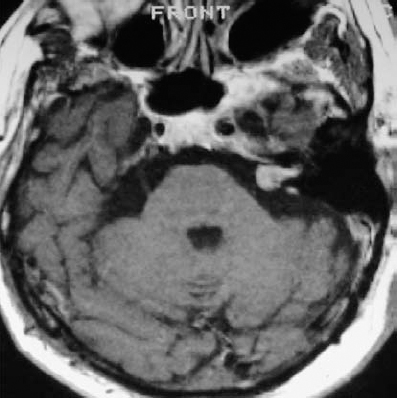
Fig. 5.25 a MRI with contrast demonstrates a grade 2 a tumor in this axial view at the level of the IAC.
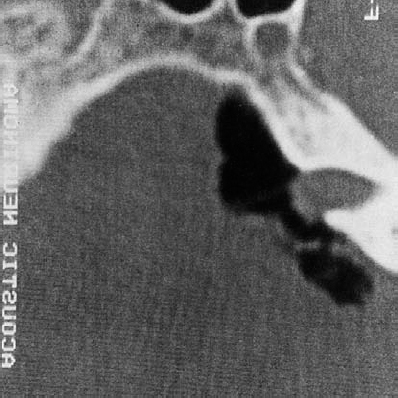
Fig. 5.25 b An air-contrast CT scan was also performed, demonstrating the tumor mass in the canal and a relative lack of bone erosion at the porus acusticus.
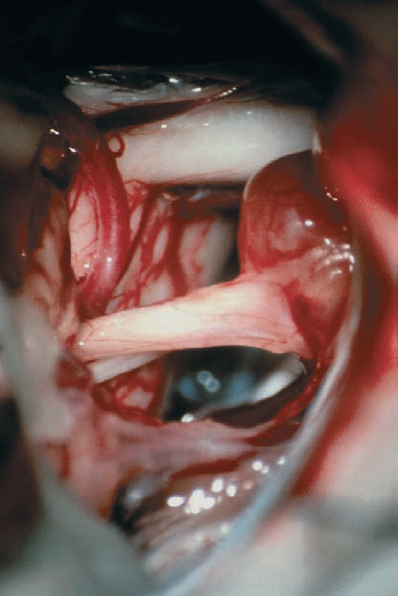
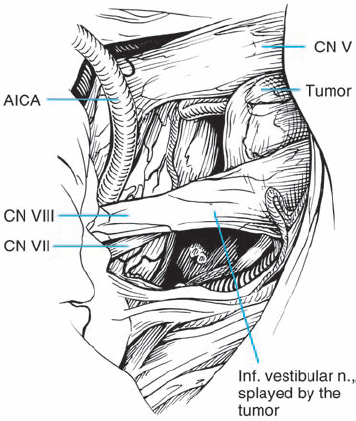
Fig. 5.25 c The initial operative view after arachnoid dissection in the CPA shows the tumor mass extending out of the porus acusticus. The facial nerve is seen at the brain stem, just inferior to the eighth nerve complex, before it passes on the medial side.
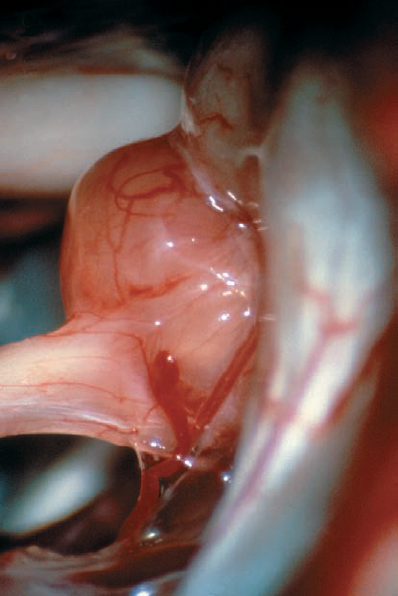
Fig. 5.25 d A higher-magnification view demonstrates the type 1 b morphology, as the tumor is deflecting the eighth nerve from the superior direction.
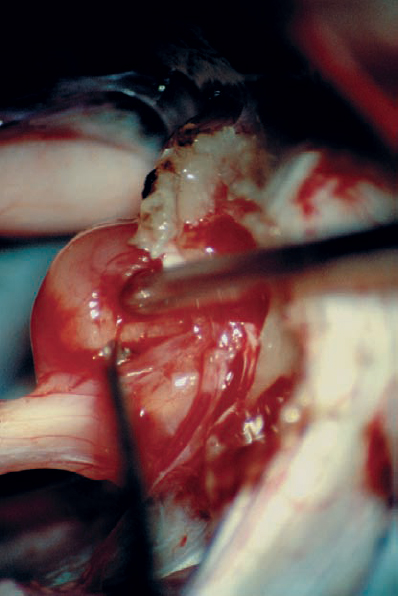
Fig. 5.25 e The dura is reflected, and limited removal of the posterior canal wall is carried out before tumor dissection. The plane between the tumor capsule and the cochlear division is carefully developed with a micro-dissector after tumor debulking.
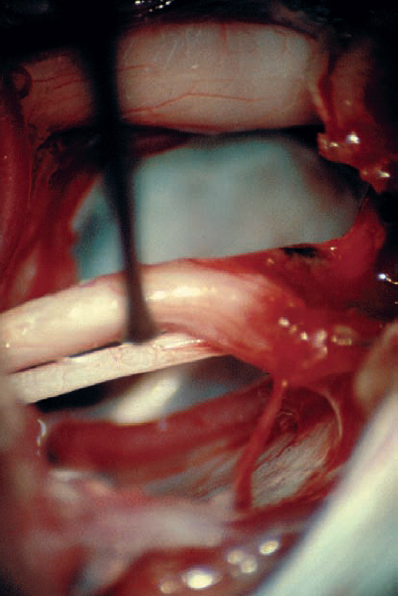
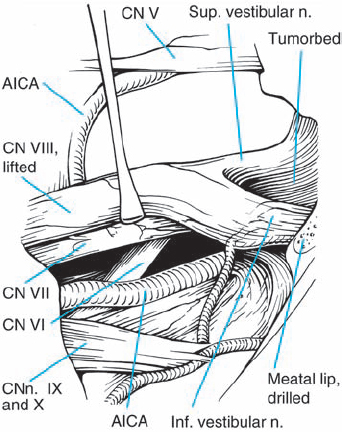
Fig. 5.25 f With a substantial amount of tumor resected, the eighth nerve complex can be freely manipulated in a limited way to reveal the course of the facial nerve and intermediate nerve on the medial aspect. The sixth cranial nerve can also be seen in the depth, as it ascends towards Dorello’s canal.
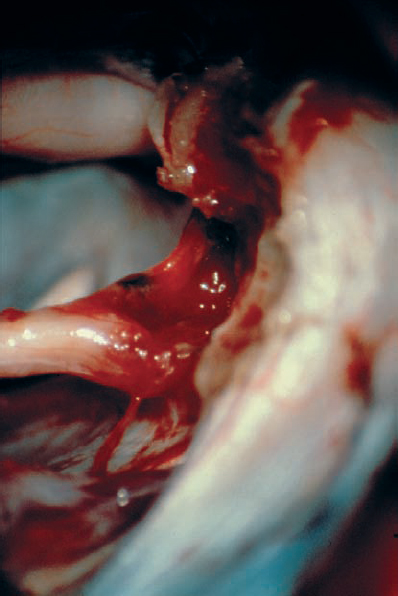
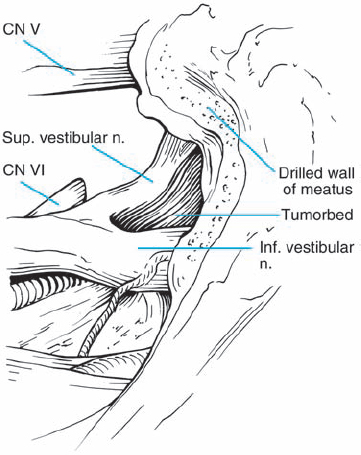
Fig. 5.25 g After tumor removal, the bloody resection bed is seen and hemostasis is carried out. It is important to never coagulate any small bleeding vessels in this area, as heat and current spread can be disastrous. A small amount of hemostatic material, such as oxidized cellulose, will suffice. The abducent nerve can be seen in the depth, entering Dorello’s canal.
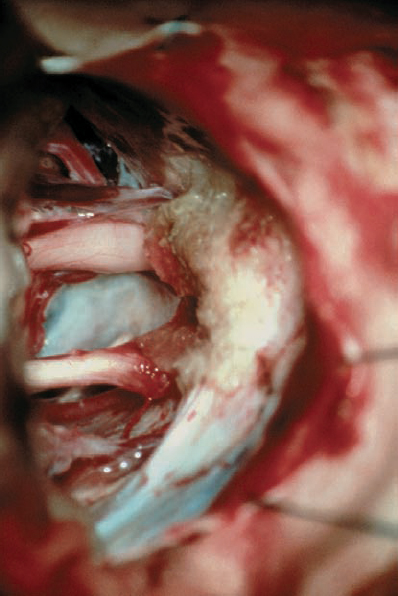
Fig. 5.25 h Before closure, hemostatic material is placed and any opened air cells are sealed in the region of bone removal.
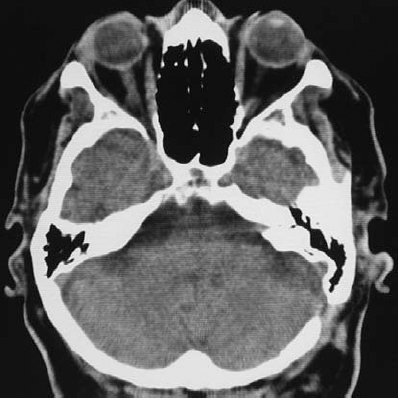
Fig. 5.25 i The postoperative contrast CT scan.
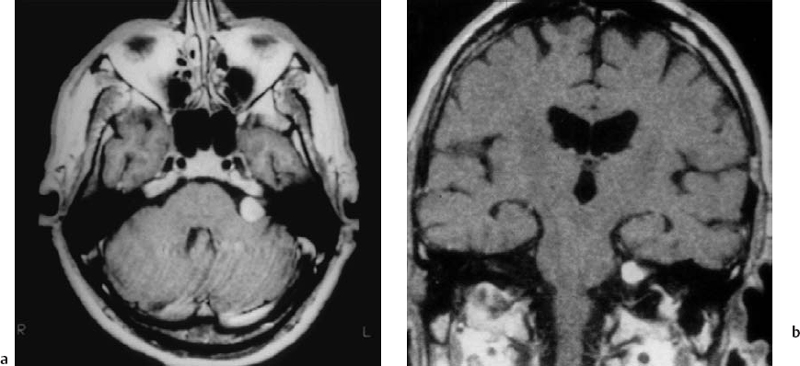
Fig. 5.26 a, b Axial and coronal T1-weighted MRIs with contrast, showing a grade 2 a tumor with very limited extension into the IAC in this patient. This is really an ideal case for removal via the retrosigmoid approach.
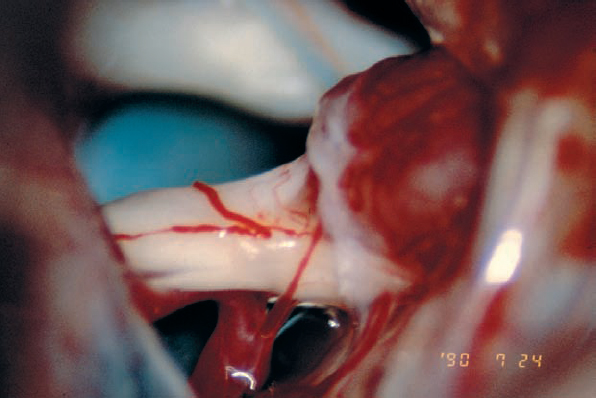
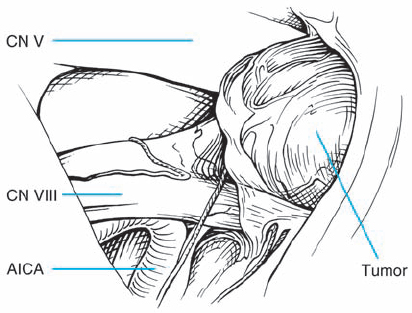
Fig. 5.26 c Exposure after arachnoid dissection reveals another case of type 1 b morphology, with the tumor mass displacing the nerve bundles from above. It appears that a very fine auditory artery is present, branching from the AICA as it turns toward the cerebellum.
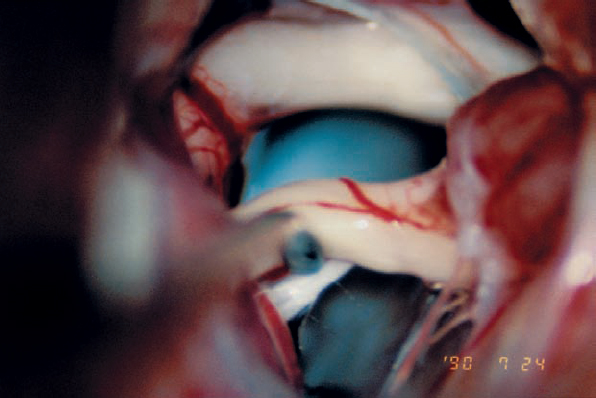
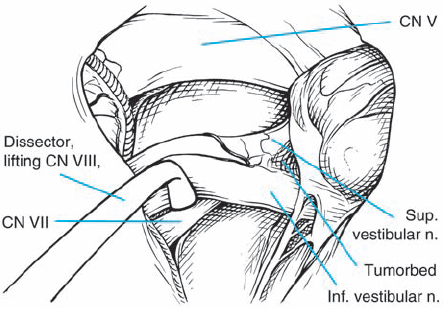
Fig. 5.26 d Gentle elevation of the eighth nerve complex near its brain-stem origin reveals the proximal segment of the facial nerve.
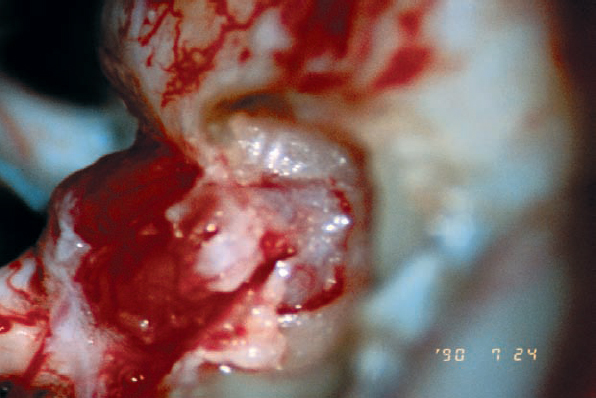
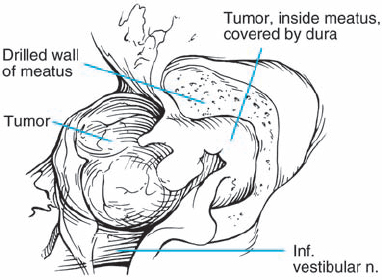
Fig. 5.26 e The tumor extension into the IAC is exposed by removal of a modest length of bone over the back wall of the IAC. In this type of case, when the extension is seen to be limited on preoperative imaging, there is little concern regarding mishaps, unless a high jugular bulb is present. In the presence of a high jugular bulb, even with such limited drilling, the risk of opening the bulb is quite high.
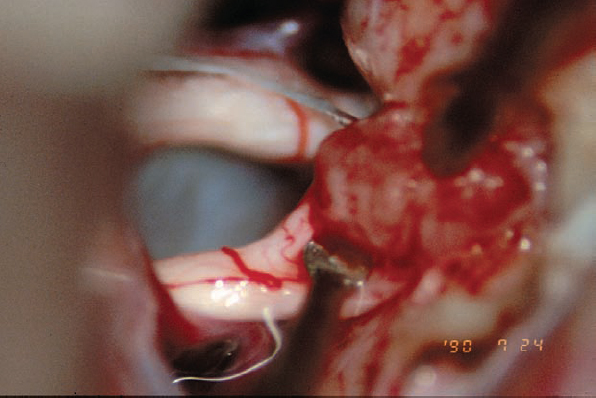
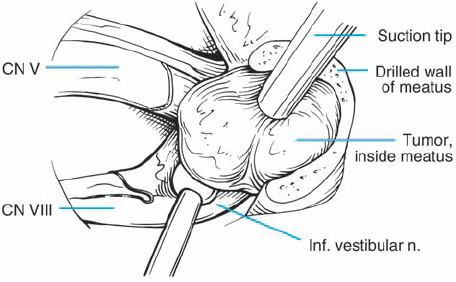
Fig. 5.26 f The tumor is debulked as the first step, and then the tumor–nerve interface is addressed. In this view, the tumor is seen retracted being with a light touch using the suction, while the tumor–nerve interface is opened with a sharp round-edged micro-dissector.
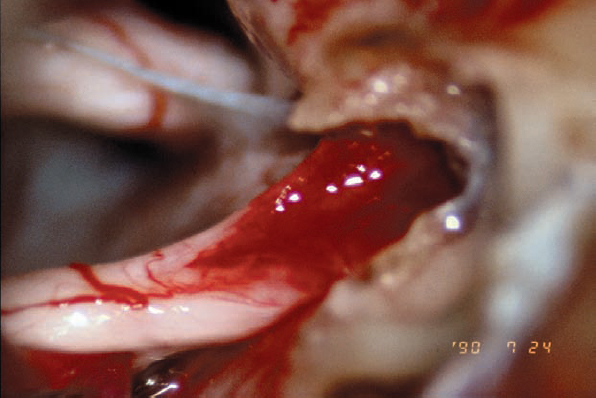
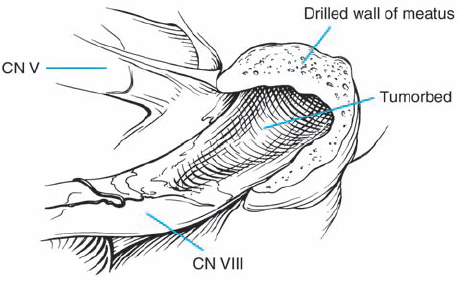
Fig. 5.26 g The hollowed-out tumor bed is left after complete removal of the mass.
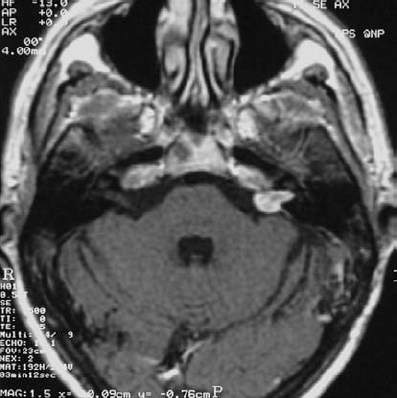
Fig. 5.27 a A contrast-enhanced axial MRI scan at the level of the internal auditory canals, showing a grade 2 a acoustic neurinoma.
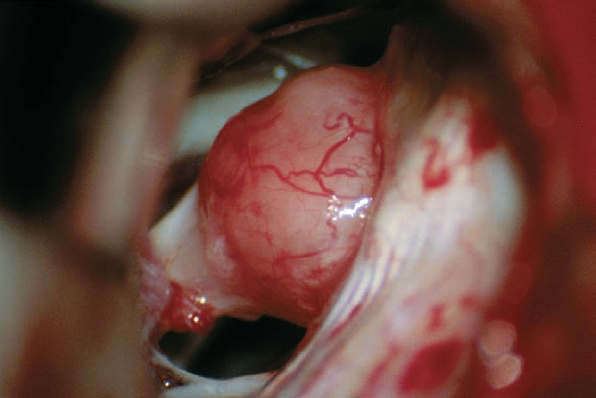
Fig. 5.27 b The intraoperative view after arachnoid dissection via a standard retrosigmoid approach demonstrates the type 2 a neurinoma protruding into the cerebellopontine angle from the porus acusticus. This exposure requires only minimal retraction of the cerebellum.
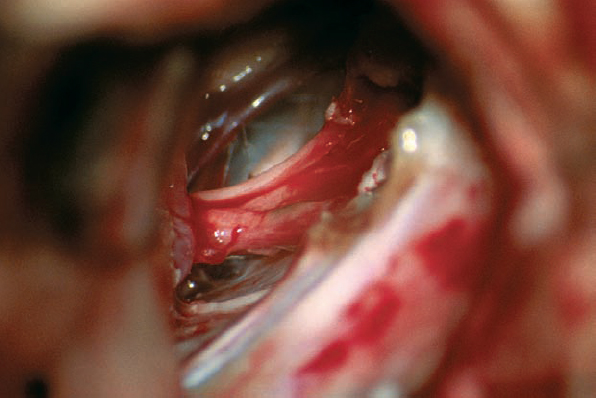
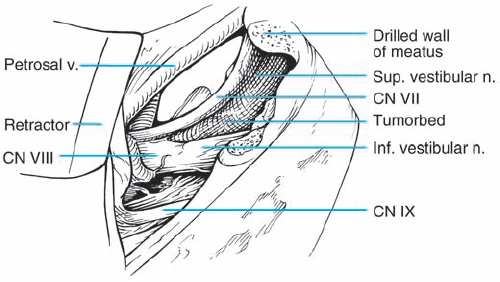
Fig. 5.27 c With the posterior wall of the IAC removed, the tumor is first debulked and then separated away from the eighth nerve complex. After removal, the facial nerve is seen here in full view, medial and superior to the eighth nerve, which is still in place.
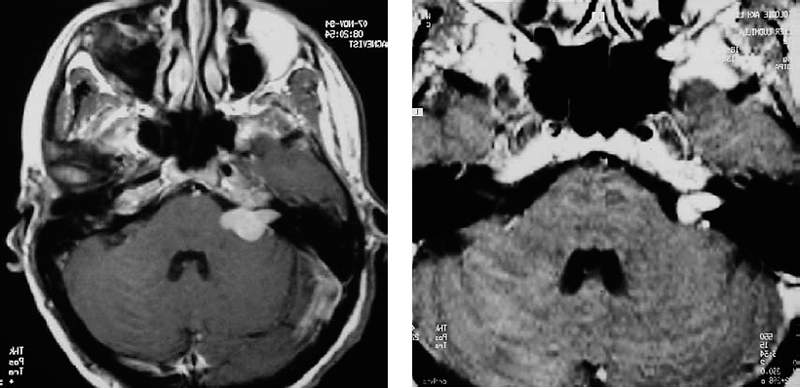
Fig. 5.28 a This axial contrast-enhanced MRI shows a grade 2 a acoustic neurinoma. The tumor in the internal auditory canal extends nearly as far as the fundus.
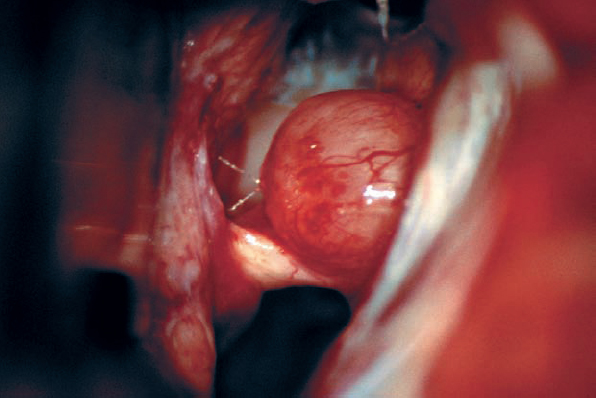
Fig. 5.28 b The initial operative view, after arachnoid dissection, shows what appears to be a type 1 b morphology, with the tumor situated above the eighth nerve bundle.
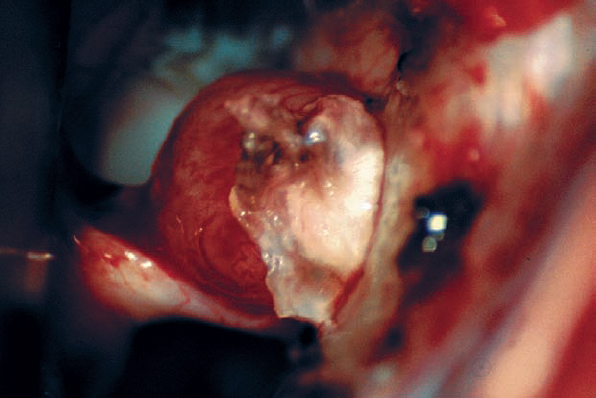
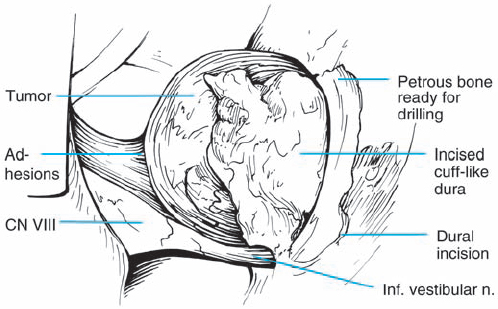
Fig. 5.28 c The dura lateral to the porus acusticus is incised and elevated, reflecting the dural flap over the tumor.
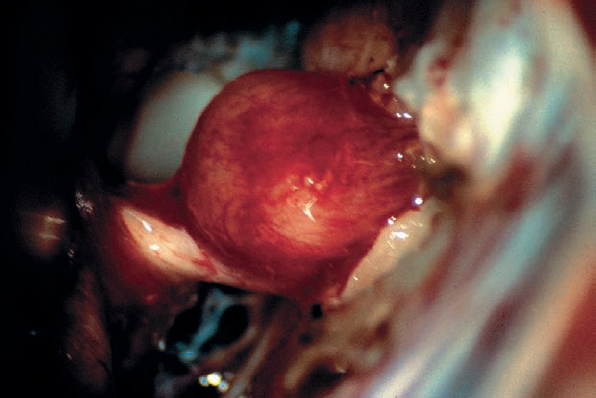
Fig. 5.28 d The posterior wall of the internal auditory canal is removed by drilling as far as possible without violating the labyrinthine structures. At this point, the tumor is ready for debulking and dissection.
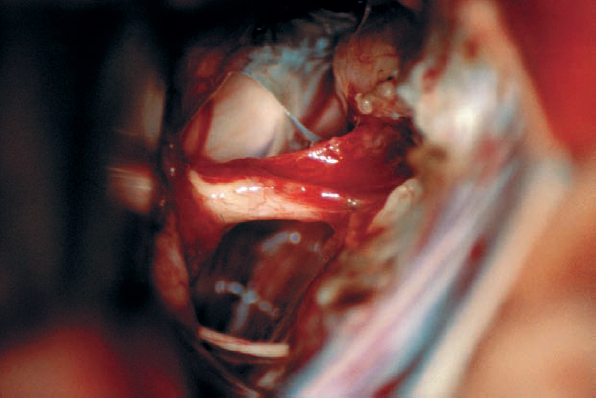
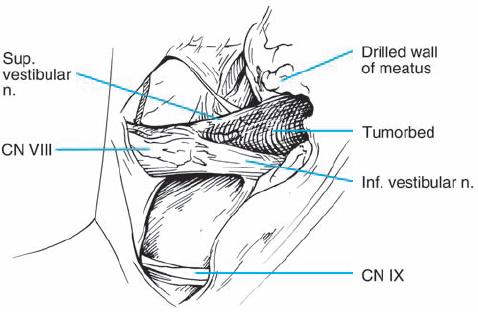
Fig. 5.28 e After removal of the type 1 b neurinoma, the resection bed is evident, with preserved eighth and seventh nerve structures.
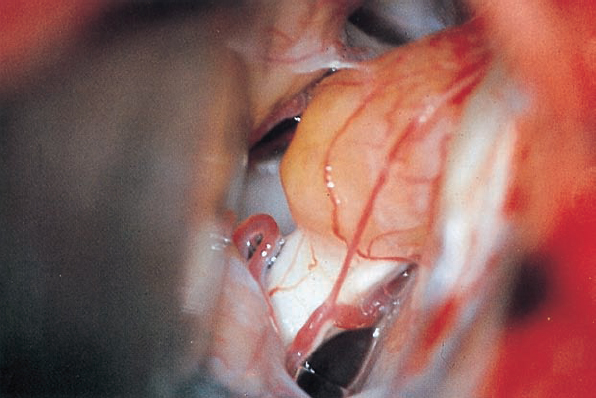
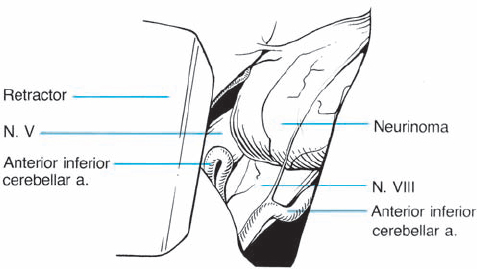
Fig. 5.29 a After the usual exposure of the meatal region, the eighth nerve, its blood supply, and the tumor emerging from the internal acoustic porus are visualized.
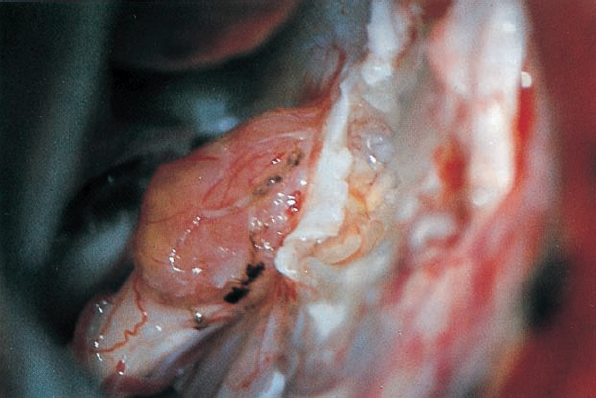
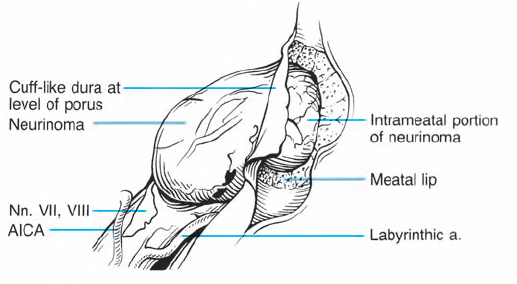
Fig. 5.29 b The dura over the meatus has been opened, and the bone removed with a diamond drill.
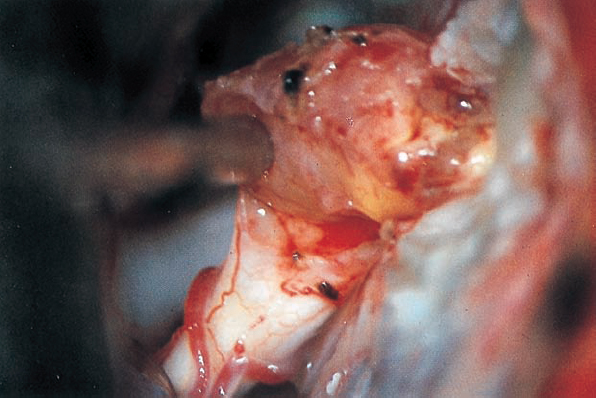
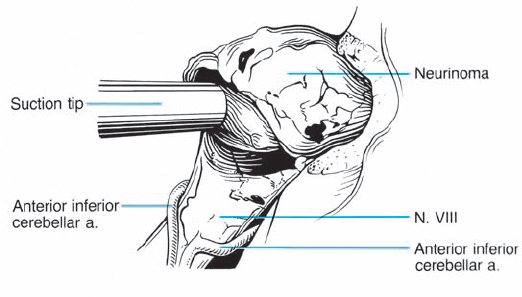
Fig. 5.29 c The tumor is carefully mobilized and sharply separated from its attachment to the inferior vestibular nerve.
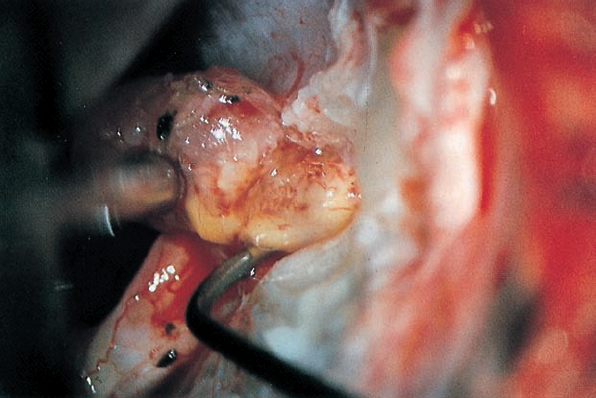
Fig. 5.29 d Using a sharp hook, a cleavage plane between the intrameatal portion of the tumor, and the eighth nerve is developed.
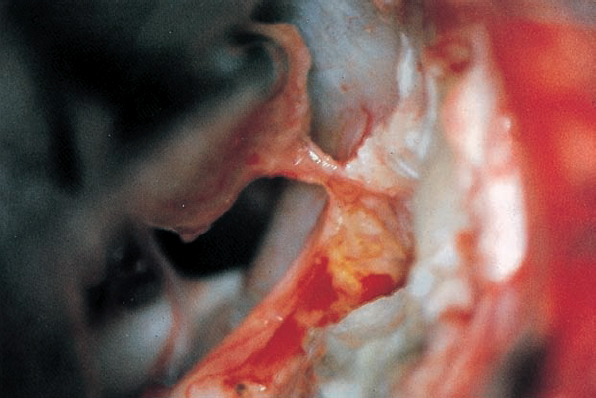
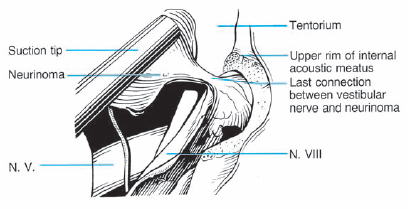
Fig. 5.29 e The distal attachment of the neurinoma to the inferior vestibular portion of the eighth nerve is visualized before being cut.
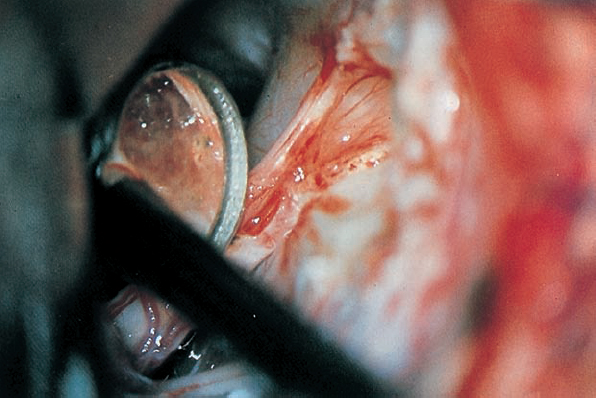
Fig. 5.29 f Inspection of the internal acoustic meatus using an angled mirror reveals a residual piece of tumor near the fundus. Nowadays rigid endoscopies are used routiniously.
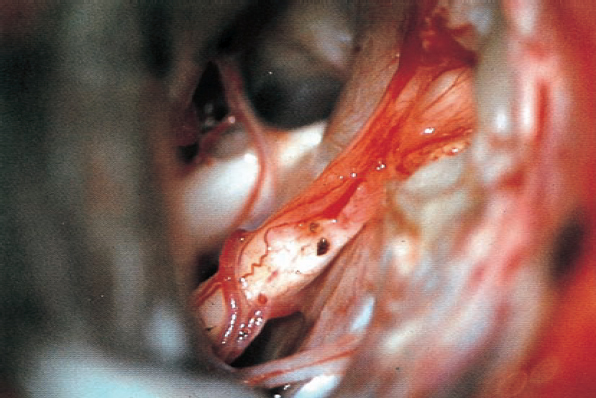
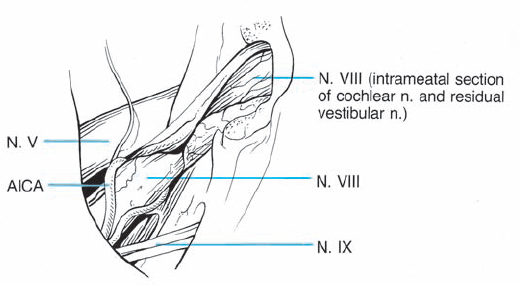
Fig. 5.29 g The continuity of the eighth nerve is seen after complete tumor removal. The patient’s hearing was preserved.
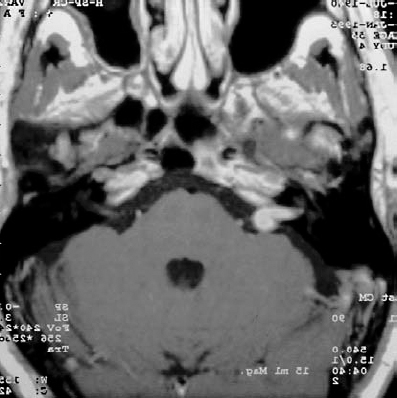
Fig. 5.30 a An axial contrast-enhanced MRI of a grade 2 a acoustic neurinoma, with extension to the fundus of the internal auditory canal.
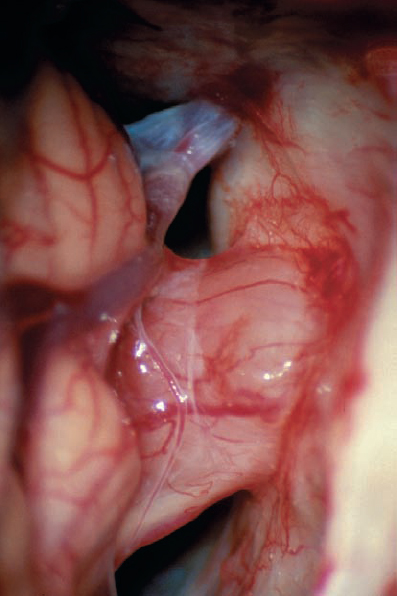
Fig. 5.30 b The initial view in a retrosigmoid approach demonstrates the neurinoma extending out of the porus acusticus in the cerebellopontine angle. The double arachnoid membrane remains over the tumor and eighth nerve complex. Superiorly, the arachnoid is seen covering the intact petrosal vein.
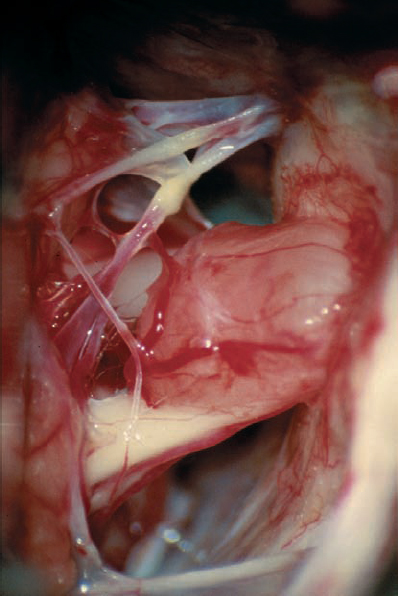
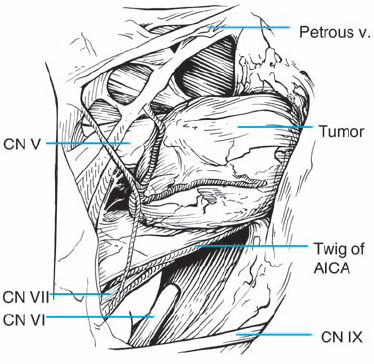
Fig. 5.30 c After arachnoid dissection, the full extent of the tumor in the cerebellopontine angle comes clearly into view. At this stage, the topographical relationship of tumor to the eighth nerve complex appears to be type 1 b. The petrosal vein complex remains covered by a layer of arachnoid and is preserved during tumor removal on principle.
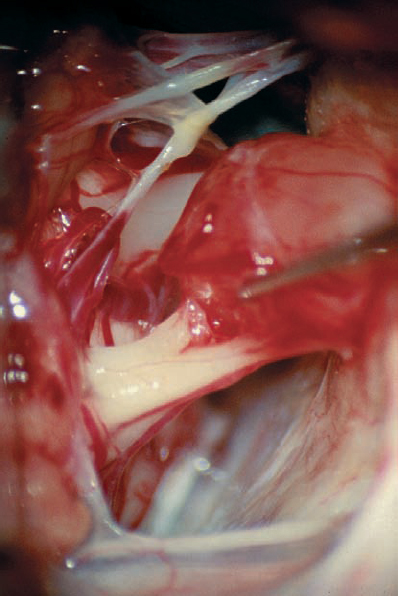
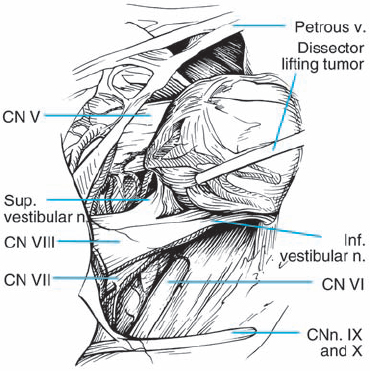
Fig. 5.30 d The plane of separation between the tumor capsule and the nerve fiber bundles medially is developed. Here the tumor is being gently elevated with a sharp hook, bringing this interface into view.
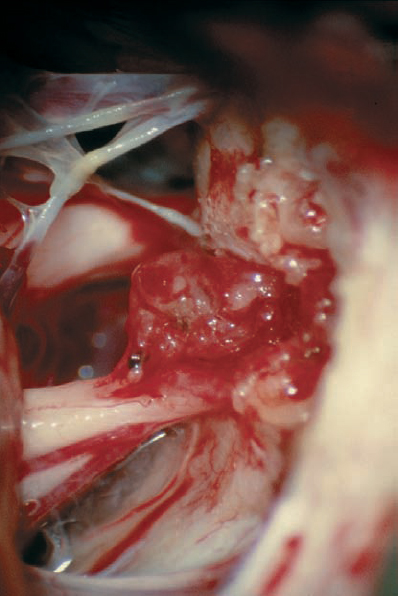
Fig. 5.30 e The posterior wall of the internal auditory canal is removed, and the dura is opened over the internal auditory canal. Internal debulking of the mass is then carried out, which is seen at an intermediate stage here. Note the preserved vascularity over the eighth nerve bundle.
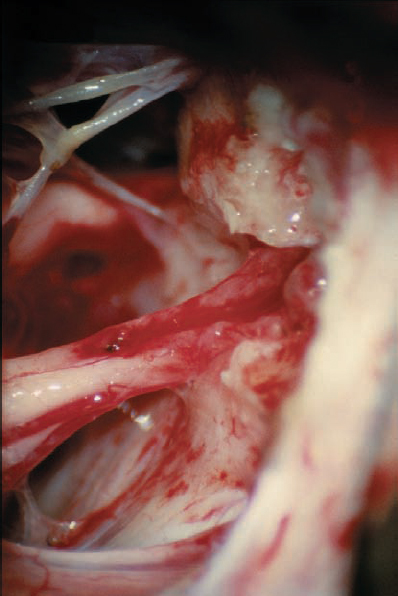
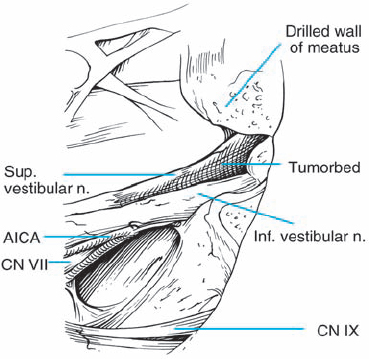
Fig. 5.30 f The tumor has been completely separated from the seventh and eighth cranial nerves. The vascularity to the cochlear nerve is preserved, as is the petrosal vein complex located superiorly.
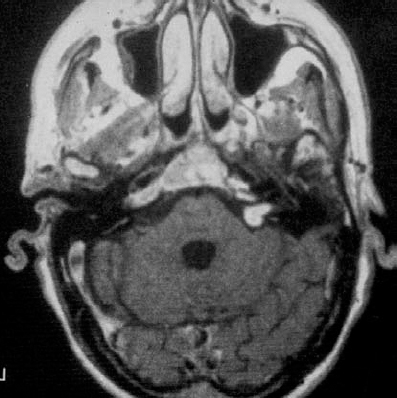
Fig. 5.31 a An axial contrast enhanced MRI of a grade 2 a tumor and its extension into the inner meatus.
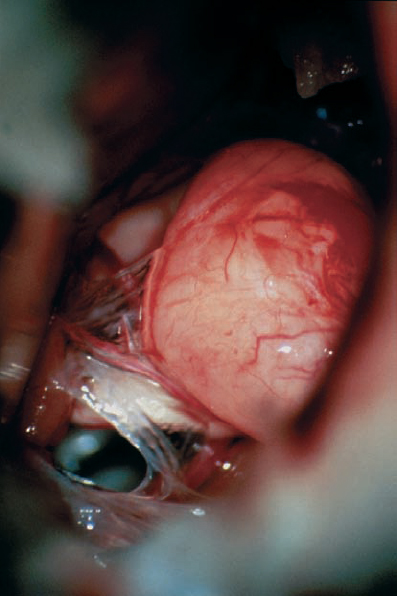
Fig. 5.31 b The initial intraoperative view after a typical retromastoid approach.
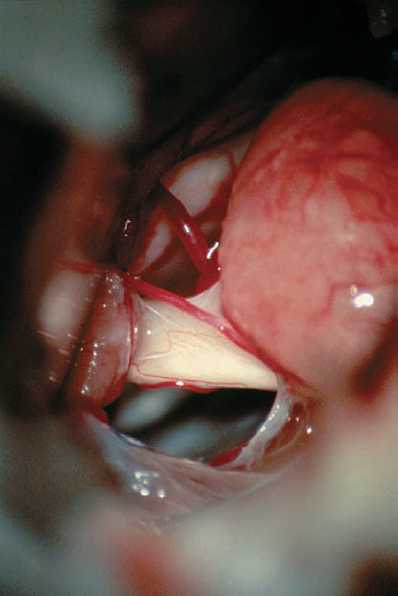
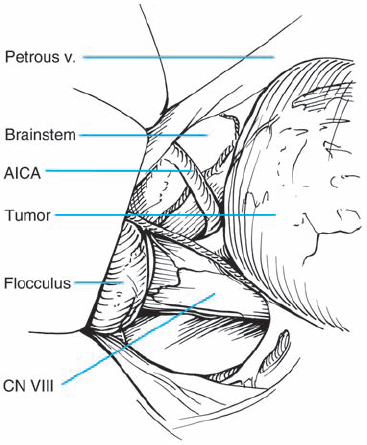
Fig. 5.31 c Higher magnification, demonstrating the vestibular cochlear bundle before the brain stem, representing a typical grade 2 a neurinoma.
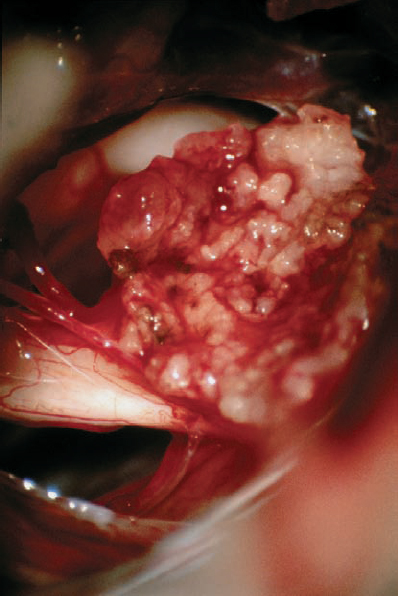
Fig. 5.31 d The situation during debulking of the tumor. A small part of the capsule has been left at the cranial nerve complex before complete resection.
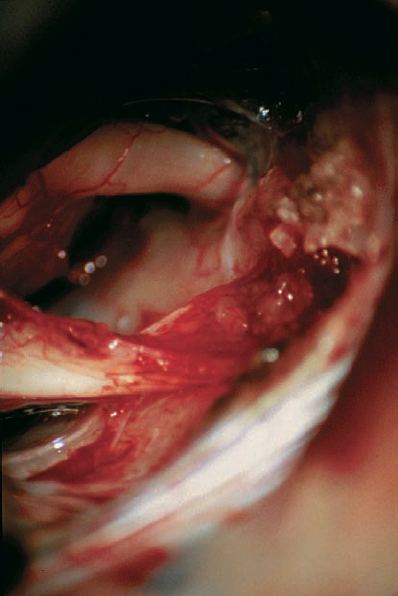
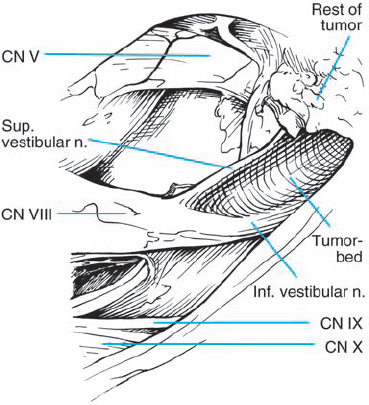
Fig. 5.31 e The situation after drilling the posterior wall of the inner meatus, showing the postoperative result. A very small part of the capsule has to be left, because it is adherent to the cranial nerve complex. In this case, it was impossible to remove these small parts of nonvital tumor tissue as well, without a risk of destroying the vestibular nerve.
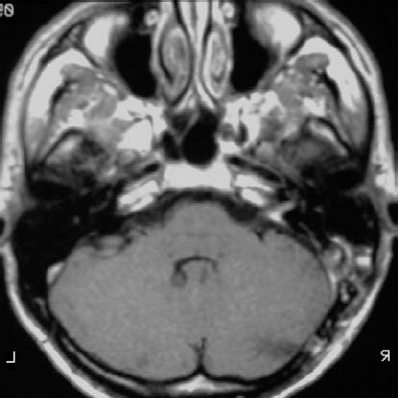
Fig. 5.31 f The postoperative contrast-enhanced axial MRI shows the mass of the tumor removed, with very slight contrast enhancement of the residual capsule that was left in place.
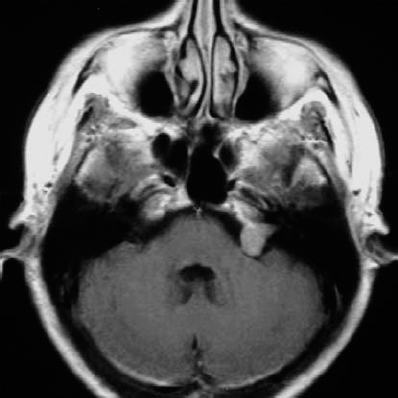
Fig. 5.32 a An axial contrast-enhanced MRI of a grade 2 a neurinoma, at the upper end of the size range for the classification.
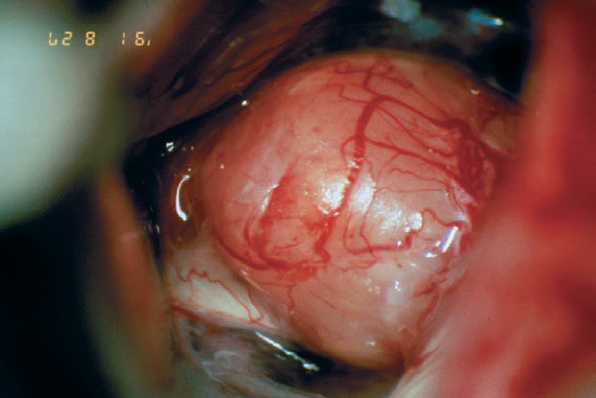
Fig. 5.32 b The initial operative view demonstrates type 1 b topography.
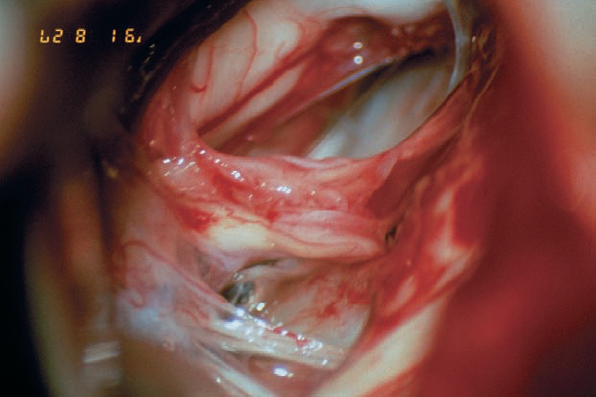
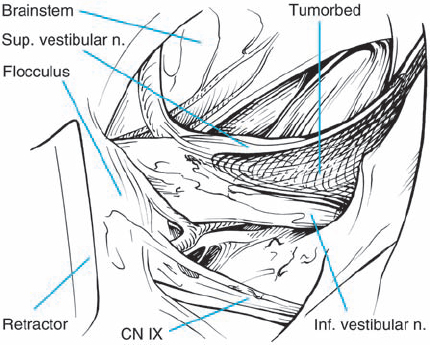
Fig. 5.32 c The tumor is completely removed, with preservation of the eighth cranial nerve and its vascularity, as well as the facial nerve.
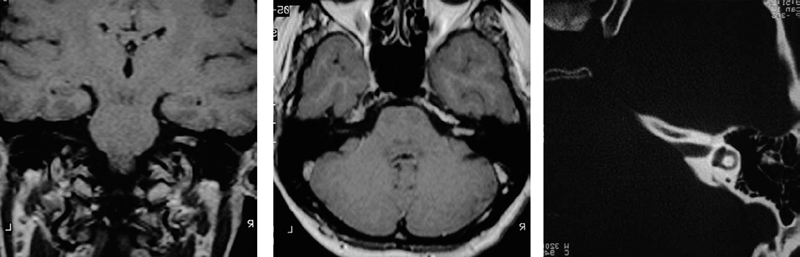
Fig. 5.33 a– c Coronal (a) and axial (b) contrast-enhanced MRI views of a grade 2 a tumor. The bone window CT scan in c shows a relative lack of bony erosion of the internal auditory canal. This section, at the level of the lateral semicircular canal, suggests that exposure of the fundus should be possible by drilling away the compact bone of the posterior wall of the internal auditory canal.
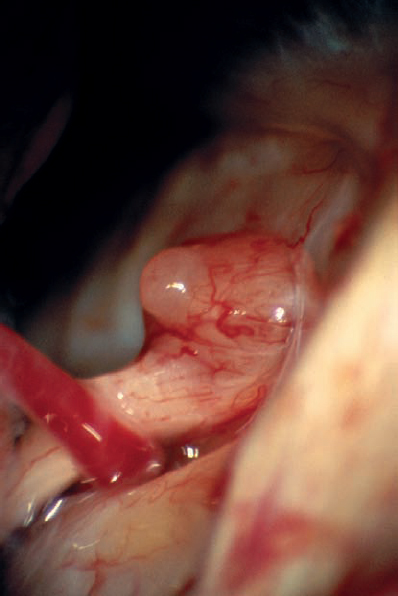
Fig. 5.33 d The initial operative view via the retrosigmoid approach shows the small protrusion of the tumor at the porus acusticus.
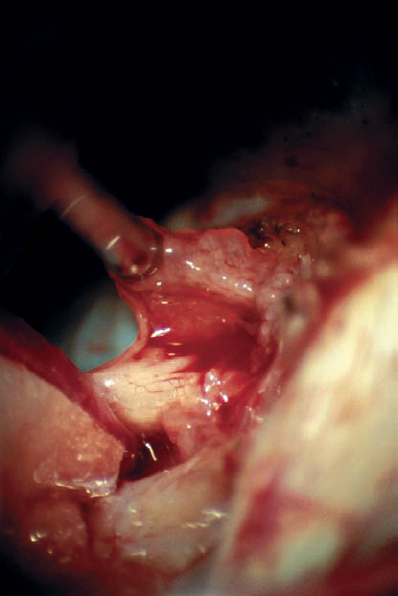
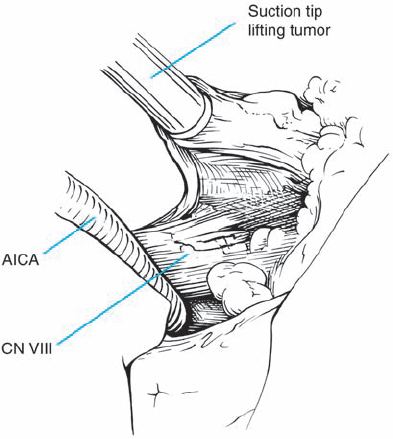
Fig. 5.33 e The interface between the tumor capsule and the eighth nerve complex is developed. The small tag of tumor is gently retracted superiorly with suction, and this small piece is removed before drilling over the posterior wall of the internal auditory canal.
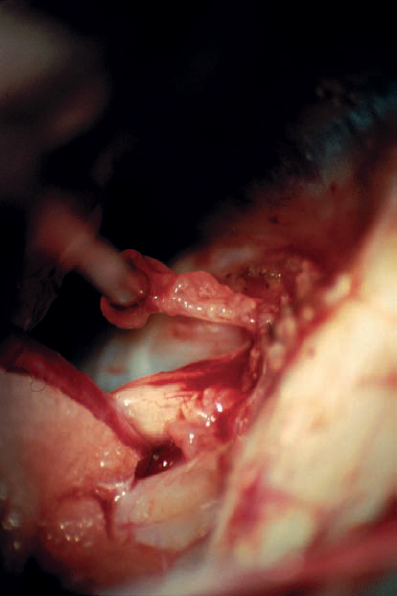
Fig. 5.33 f The tumor is further removed and followed into the internal auditory canal.
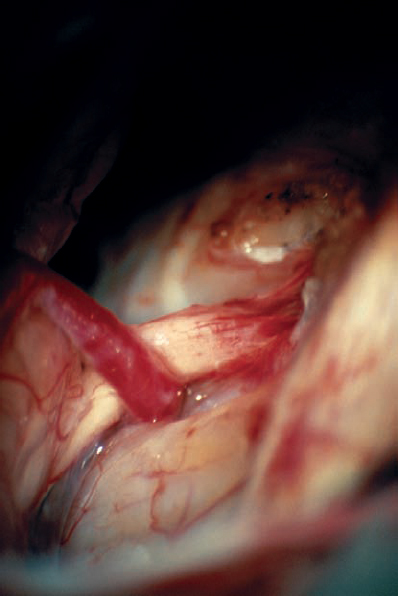
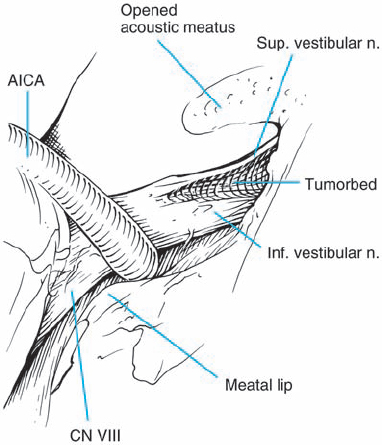
Fig. 5.33 g The posterior wall of the internal auditory canal is being drilled. Not visible here is the high-riding dome of the jugular bulb, which prevented full exposure of the canal.
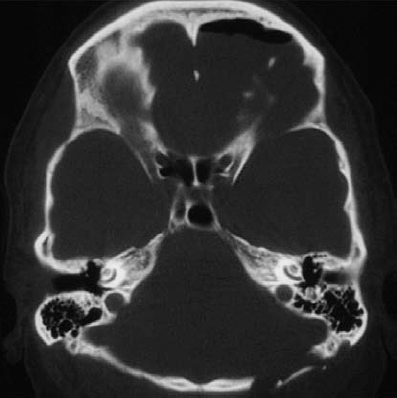
Fig. 5.33 h An axial bone window CT scan at a slightly lower level than in c, demonstrating the high-riding dome of the jugular bulb. A high jugular bulb is estimated to occur in 5– 10% of patients. In these cases, it is advisable to obtain a fine-cut bone window CT scan preoperatively, to detect the presence of this anatomic variation.
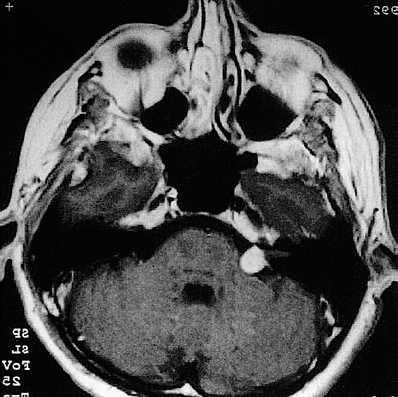
Fig. 5.34 a The axial CT demonstrates a grade 2 acoustic neurinoma. The striking feature in this patient was total hearing loss with such a small tumor. The patient also reported constant tinnitus.
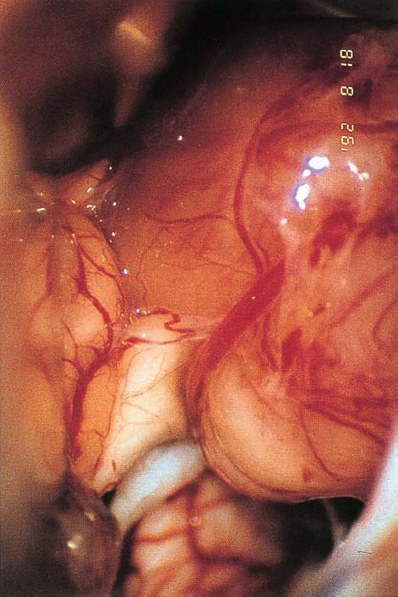
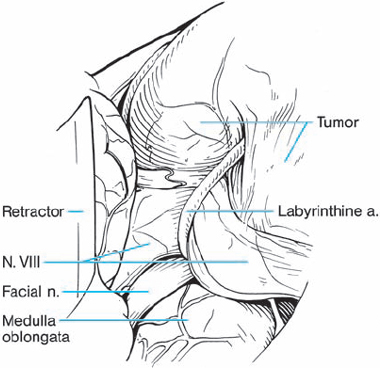
Fig. 5.34 b Using a right retromastoid approach, the neurinoma becomes visible. The eighth nerve follows a course along the inner surface of the tumor and appears severely compressed by the labyrinthine artery, which traverses the nerve shortly after its origin from the brain stem.
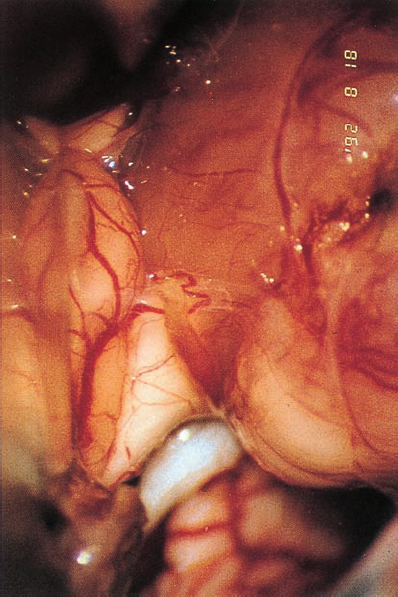
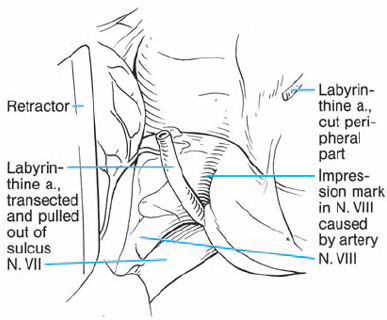
Fig. 5.34 c The labyrinthine artery is sectioned and mobilized from the eighth nerve.
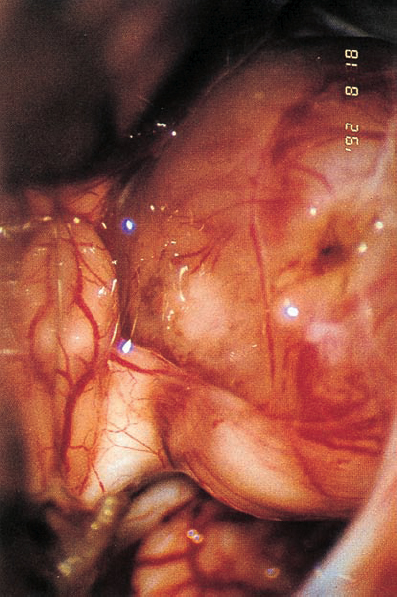
Fig. 5.34 d The eighth nerve is separated from the tumor, and a groove remains from the vascular compression.
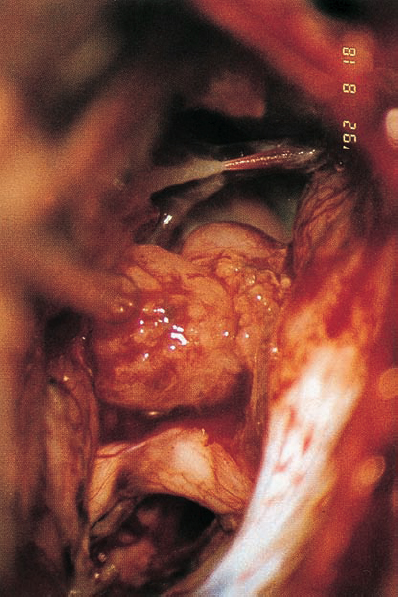
Fig. 5.34 e The tumor is mobilized from the internal auditory meatus without drilling of the posterior meatal wall.
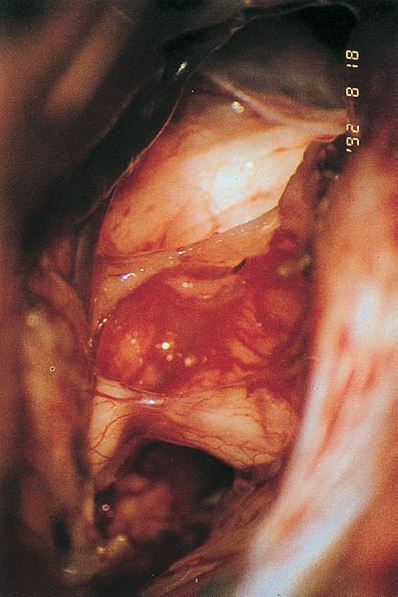
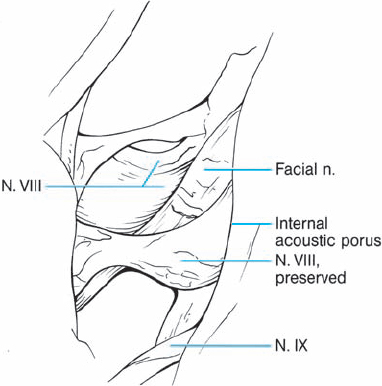
Fig. 5.34 f After the neurinoma has been completely removed, the intact seventh and eighth nerves can be seen.
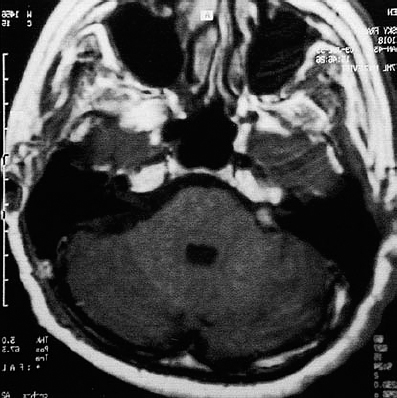
Fig. 5.34 g The postoperative contrast-enhanced MRI shows a slight enhancement at the extrameatal portion of the cranial nerve complex, but no intrameatal enhancement. The development of scar tissue can be expected after the operation.
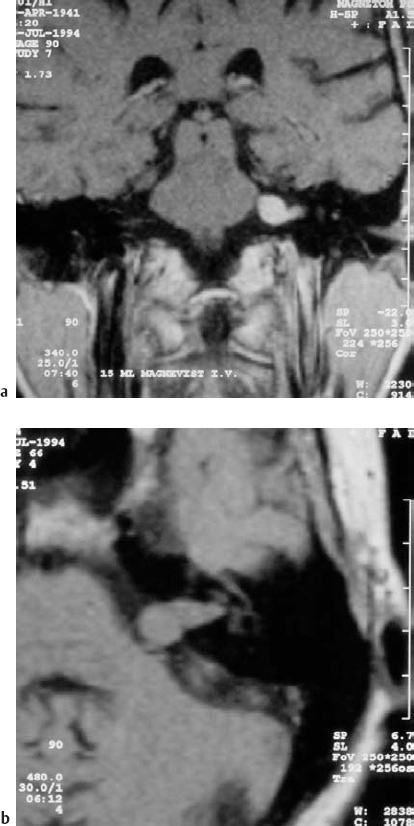
Fig. 5.35 a, b Preoperative contrast-enhanced coronal (a) and axial noncontrast (b) images of a grade 2 a acoustic neurinoma.
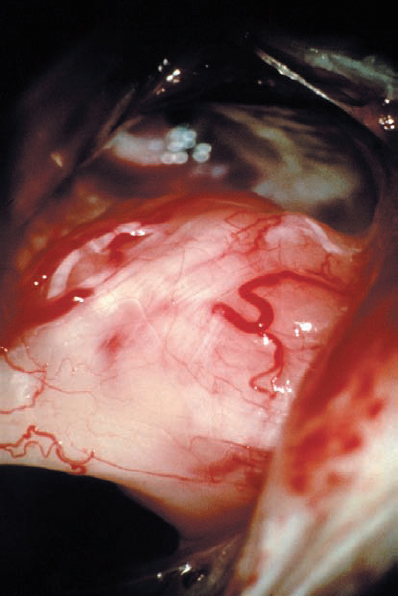
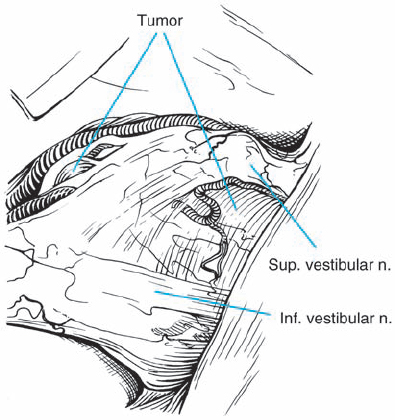
Fig. 5.35 c A microscopic intraoperative view via a retrosigmoid approach, demonstrating what appears to be type 2 a topography, with the mass of the tumor separating the eighth nerve bundles from the facial nerve. It is possible to see the eighth cranial nerve thinly fanned out over the lateral aspect of the neurinoma.
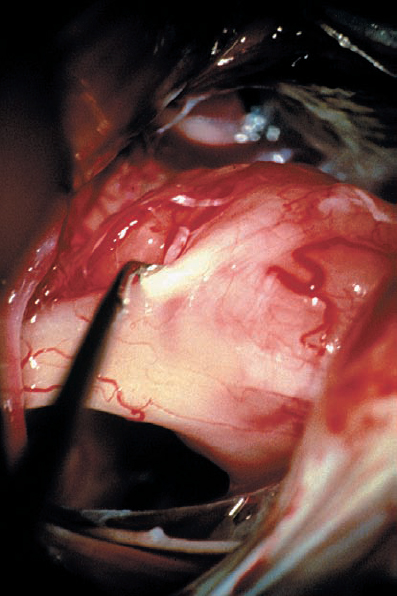
Fig. 5.35 d The fiber bundles of the eighth cranial nerve can be carefully separated from the bulk of the tumor mass. Here, an angled sharp round microdissector is being used to develop the plane at the tumor–nerve interface.
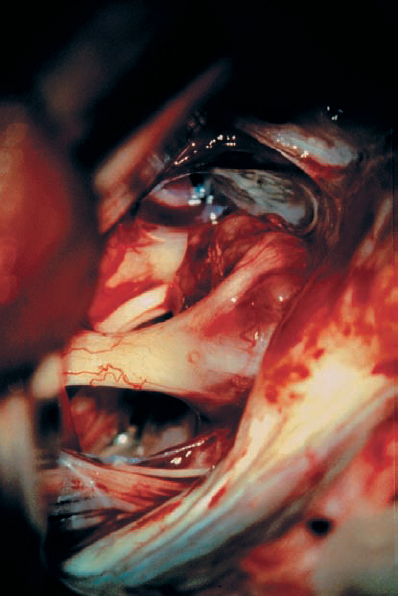
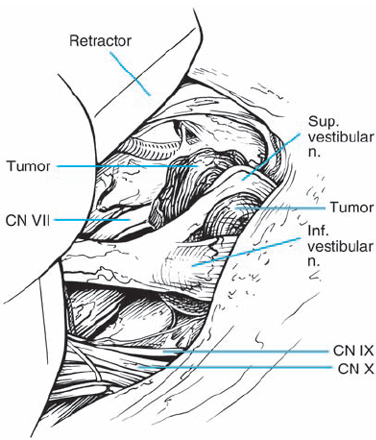
Fig. 5.35 e The posterosuperior aspect of the tumor has been opened and internal debulking has been carried out, resulting in a reduction in the overall tumor mass. The nerve fiber bundles are now more clearly defined over the lateral aspect of the mass as it enters the porus acusticus. The facial nerve is now better seen passing medial to the mass of the tumor.
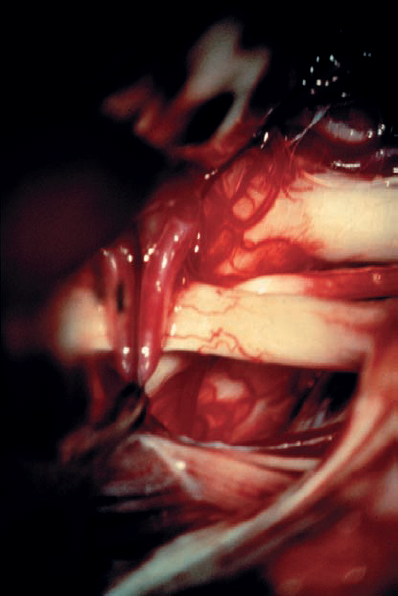
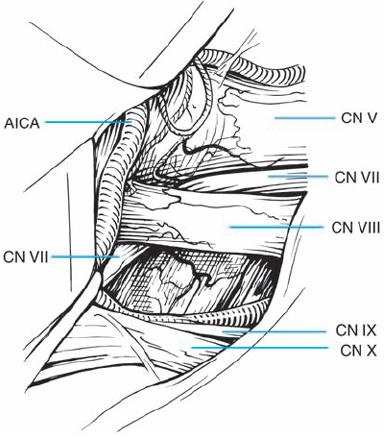
Fig. 5.35 f After tumor removal, the relationships of the preserved cranial nerve structures are in full view. The origins of the seventh and eighth cranial nerves at the brain stem are exposed. There is a loop of the anterior inferior cerebellar artery (AICA) passing lateral to the eighth nerve origin at the brain stem.
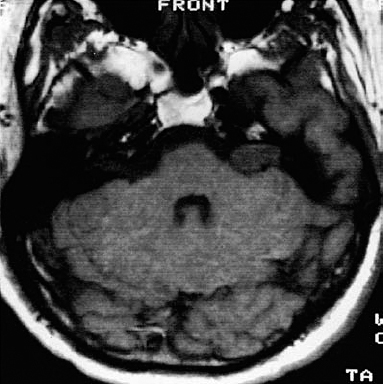
Fig. 5.36 a An axial contrast-enhanced MRI scan, showing a typical grade 2 a neurinoma.
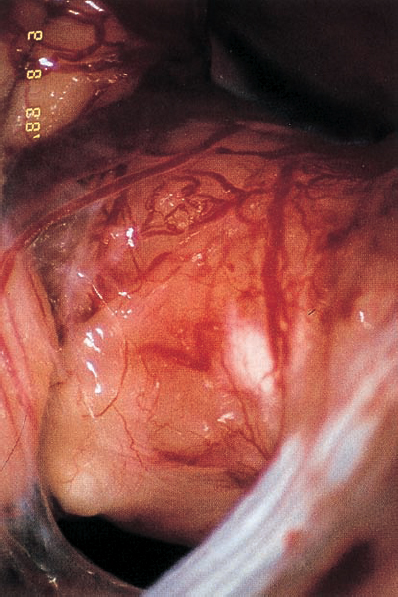
Fig. 5.36 b Using a right retromastoid approach, a large acoustic neurinoma covered with arachnoid is encountered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree