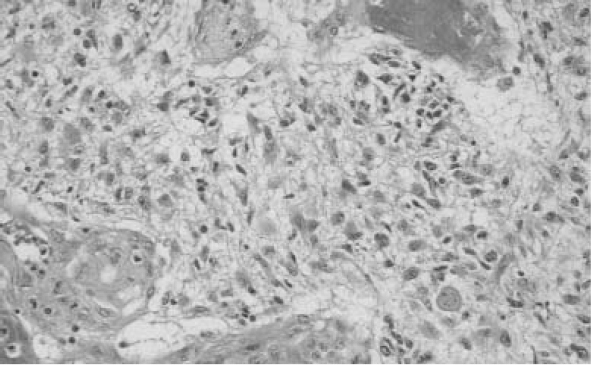
Over 125 years have passed since the historic report of Bennett and Godlee1 in 1888 describing their initial surgical approach to a patient with a primary brain tumor; yet, the surgical aspects of malignant glioma remain an area of discussion and controversy. The role of surgical intervention continually evolves as new techniques become available and continued study outlines the effectiveness and contributions of surgical procedures for survival and quality of life for patients with malignant glioma. Controversy has arisen partly because of the lack of any consistent effective therapeutic intervention. Numerous factors have been proposed as contributors to the overall outcome in this patient population. Primary malignant brain tumors are among the most difficult human malignancies to treat. In contrast to small metastatic lesions to the brain, small malignant primary tumors often progress rapidly despite multi-modality therapy. A recent review estimated 18,400 cases of central nervous system cancer will occur in 2004; 12,690 deaths will be attributed to the tumors.2 The majority of these primary central nervous system cancers are malignant gliomas. Despite recent evidence demonstrating improved survival over the past 30 years, the possibility of long-term survival remains remote and treatment decisions are focused on neurocognitive and quality-of-life outcomes.2 In many instances, the main bulk of the tumor is managed differently than the remaining rim of infiltrating malignant cells that are invariably present. A significant challenge remains in developing therapies targeted at this infiltrative component. This residual area, usually outside the zone of enhancement, is the site of local recurrence and is more likely to cause problems with long-term control. In our research, we have used the National Library of Medicine’s MEDLINE database. Additional resources, however, for evidence-based neuro-oncology studies include the Cochrane Reviews (available through their Web site at www.cochrane.org) and Neurosurgical Focus, which has published evidence-based articles and guideline development projects of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons (AANS/CNS) Joint Section on Tumors. Neurosurgical Focus is available through their Web site at www.aans.org/education/journal/neurosurgical. The diagnosis of a malignant glioma continues to be made on histopathologic analysis; tissue obtained either at the time of biopsy or cytoreductive surgery is evaluated (Fig. 3–1). To evaluate the diagnostic accuracy in patients with malignant glioma, we initiated a MEDLINE review of the medical literature using the keyword glioma and cross-referenced with biopsy, accuracy, diagnosis, variability, and pathology. Initially, we reviewed the titles and abstracts to remove duplicates and eliminate erroneous entries; we then scanned bibliographies of selected articles for additional relevant references. We identified numerous studies on noninvasive imaging techniques for refining the diagnosis, but our focus in this chapter is on those studies addressing the actual analysis of the tissue. We selected several articles that specifically cover the techniques of diagnosing malignant glioma and outline them below. The studies include issues of observer variability, the contribution of cytologic techniques, and the role of image analysis (Table 3-1). Mittler et al3 reported an evaluation of observer reliability in the histologic grading of glial tumors. In this study, four neuropathologists under-went a blinded review of 30 brain biopsy specimens. Both intraobserver and interobserver agreements were analyzed. Intraobserver agreement was 75% for glioblastoma (GBM) and 51% for anaplastic astrocytoma (AA) with a mean intraobserver agreement of 63.9% (kappa = 0.50). Interobserver agreement was 62% for glioblastoma (kappa = 0.39) and 36% for AA (kappa = 0 .06). The authors pointed out the considerable discrepancy observed, not only between different pathologists but also between interpretations of the same pathologists at different times. Figure 3–1 Histologic section of glioblastoma. A subsequent blinded study by Prayson et al4 compared the grading consistency of five neuropathologists with five surgical pathologists in retrospectively evaluating 30 biopsy specimens of normal brain and varying grades of glial tumors. Agreement among the neuropathologists was complete (five of five) in 12 of 30 cases (40%), and four of the five agreed in 26 of 30 cases (87%). In contrast, there were only six cases (20%) in which the surgical pathologists all agreed, 13 cases (43%) where four of the five surgical pathologists agreed. At least two disagreed with the rest of the group in 50% of the cases. The kappa value for interobserver variability among the neuropathologists was reported as higher than that for the surgical pathologists (kappa = 0.63 versus 0.36). From the data provided in the article, the kappa value for agreement on the diagnosis of glioblastoma among the neuropathologists was calculated to be 0.81 versus 0.63 for the surgical pathologists and 0.88 versus 0.55 for AA. The authors conclude that experience in grading glial tumors is an important factor in reliably establishing the diagnosis, and that it can be accomplished in a high percentage of cases. Additional observation suggested agreement on the diagnosis of the malignant tumors (GBM and AA) is less variable than in the entire spectrum of glial neoplasms included in this study. Roessler et al5 retrospectively reviewed 4172 intraoperative cytologic smears from intracranial procedures (3541 open procedures and 631 stereotactic biopsies with a wide variety of diagnoses) and compared preliminary versus final pathological diagnosis. Complete correlation was obtained in 90%; the correlation in the diagnosis of glioblastoma was reported as 95.7% with a calculated sensitivity of 0.97 and specificity of 0.99. Significantly less diagnostic accuracy was achieved with lower-grade glial tumors (oligodendroglioma = 81% and ependymoma = 78%). The authors conclude that cytologic smears have a high diagnostic accuracy when compared retrospectively with the final histopathologic diagnosis in the majority of cases and are particularly accurate in high-grade glial tumors. In this study, the glioblastoma group appeared to include all malignant glial tumors (combining both AA and GBM).  3
3 
Neuro-Oncology: The Role of Surgery in the Management of Malignant Glioma
Timothy C. Ryken, Bruce Frankel, Mark Bernstein
Patient Assessment
Reliability of Histologic Diagnosis of Malignant Glioma
| Reference | Study Design | Data Class | Conclusion |
|---|---|---|---|
| Prayson et al4 | Study comparing the diagnoses of five neuropathologists with five surgical pathologists assigning AST grade in 30 neoplastic and non-neoplastic lesions | I for the neuropathologists III for the surgical pathologists | Neuropathologist agreement 5 of 5 in 12 cases (40%), 4 of 5 in additional 14 (46.7%), and 3 of 5 (10%) in 4 cases. Surgical pathologist agreement 5 of 5 in 6 cases (20%), 4 of 5 in additional 7 cases (23.3%), 3 of 5 in 17 cases (56.7%). The main discrepancy in both groups was determining reactive gliosis from infiltrating tumor. Kappa agreement among neuropathologists was 0.63 and in the surgical pathology group was 0.36. This study indicates the impact of experience in grading primary brain tumor histopathology and suggests that even among experienced neuropathologists significant variability is likely. |
| Marie et al10 | Study examining 50 astrocytic gliomas of varying grades to examine the interobservor variability of MIB-1 labeling indices by six independent neuropathologists | II | Interobservor variability was high. The best results were with a cut-off value of 5.0% yielding a pairwise kappa value ranging from 0.52 to 0.80. Unclear what the overall kappa agreement reported is, but the implication is that significant interobservor variability is problematic for MIB-1 labeling interpretation. The authors conclude that the high level of interobserver variability suggests that MIB-1 LI prognostic cut-off values may not be useful clinically for predicting outcome in individual patients with primary brain tumors and that further prospective studies are needed investigating the prognostic usefulness of MIB-1 LI ranges that optimize interobserver agreement. |
| Mittler et al3 | 30 AST evaluated by four neuropathologists examined to determine specimens obtained interobserver and intraobserver variability in two sessions separated by 5 to 14 weeks | III | Intraobserver agreement was 74.73% for GBM, 51.43% for AA, and 65.22% for low-grade AST. The most common disagreements were between AA and GBM, followed by disagreements between anaplastic and low-grade AST. Interobserver agreement on initial readings was 62.41% (kappa = 0.39) for GBM, 36.04% (kappa = 0.06) for AA, and 57.14% (kappa = 0.48) for low-grade AST. A significantly greater degree of reliability was seen in histopathological diagnoses of low- or high-grade AST than in those of intermediate-grade AST. Excellent study design but the kappa values reported are low. The authors conclude that the highest variability occurs in intermediate-grade tumors and that the considerable variability is an issue that needs to be further addressed by analysis of current and proposed AST grading schemes. |
| Decaestecker et al13 | Image analysis study of 250 astrocytic tumors: AST = 39 patients AA = 47 patients GBM = 164 patients | III | The authors propose a methodology to improve the accuracy of the prognostic values associated with conventional morphologically based classifications in adult supratentorial astrocytic tumors. Descriptive quantitative variables (related to DNA ploidy level and morphonuclear aspects) were generated by means of computer-assisted microscopy and the data analyzed based on an artificial intelligence–related method (the decision tree approach). The authors propose that computer-aided methodology could improve prediction of prognosis when compared with conventional morphologically based classifications by providing intermediate reference points on the biological continuum of astrocytically based tumors. Kappa values not presented. |
| Gaudin et al9 | Study integrating cytopathologic and histopathologic techniques in 74 stereotactic biopsies. Diagnostic accuracy was assessed by determining whether classification of the biopsies as gliosis, AST, AA, or GBM predicted survival. | II | Four cases (5%) were classified as gliosis, 7 (9%) as atypical gliosis, 4 (5%) as high-grade mixed oligodendroglioma/AST, 11 (15%) as AST, 21 (28%) as AA, and 27 (36%) as GBM. Median survival was 11 months in patients with OA, 57 months in patients with AST, 10 months in patients with AA, and 5 months in patients with GBM. Diagnosis based on crush preparations made during the procedure was highly correlated with the final diagnosis and survival. Specifically in multivariate analysis: age greater than 55 (p < 0.0001, hazard ratio = 3.58), nuclear atypia (p = 0.004, hazard ratio = 4.30), necrosis (p = 0.016, hazard ratio = 2.14) using a Cox proportional hazards model. The authors conclude that the diagnosis of stereotactic brain biopsies using cytopathology with onsite evaluation in combination with histopathological evaluation of needle cores is accurate based on a survival analysis. Astrocytoma versus gliosis can be difficult to distinguish. |
| Revesz et al11 | Review of 273 patients with gliomas comparing pathological features and survival [data available on 209 patients (77%)] | II | Glial tumors graded using Kernohan criteria, Daumas-Duport,11 and survival compared with specific pathological features. Findings suggested that both the Kernohan and Daumas-Duport classification systems were significantly correlated with survival (Cox analysis, p < 0.0001), but the Kernohan did not differentiate between grades III and IV, whereas the Daumas-Duport did. Mitosis (p < 0.0001, x2 = 17.9), endothelial proliferation (p < 0.0001, x2 = 39.4), and necrosis (p = 0.0007, x2 = 11.5) were all significantly correlated with survival. |
| Martin et al12 | 272 gliomas (GBM = 70; 91 AST = 91; pilocytic AST = 56; oligodendrogliomas = 55) were examined with automated microscopic image analysis. III | III | Thirteen morphometric-densitometric parameters of tumor cell nuclei were tested together with two mitotic parameters. Objective and reproducible data on numerical nuclear density, nuclear size, nuclear shape, optical density, and mitotic activity of the gliomas were obtained from the morphometric–densitometric parameters. All gliomas but GBs were subdivided by four tumor grades. The morphometric-densitometric and mitotic data recorded were statistically compared to tumor-grade numerical nuclear density, deformation of nuclei, and mitotic activity were found to increase significantly with increasing tumor grade (Student’s t-test, Wilcoxon’s test, alpha = 0.05). The data obtained were proposed for use as reference values for objective, reproducible automatic glioma grading. |
| Coons et al6 | 244 glioma pathological specimens reviewed by four observers in four sessions to determine interobserver concordance rates and compared with an additional 315 gliomas with known survival data as a validation study | II | Significant improvement in diagnostic concordance with each session was observed (p = 0.02). For the first session, the concordance rates were as follows: all four reviewers, 52%; any three reviewers, 60%; two reviewers, 70%. Improving by the fourth session to 69%, 75%, and 80%, respectively. Kappa values were 0.66, 0.71, 0.76, and 0.82. The highest discrepancy related to the classification. Improvement related to the refinement of criteria distinguishing diffuse AST from oligodendrogliomas/oligoastrocytomas and pilocytic AST. The authors conclude that oligodendroglial tumors may comprise up to 25% of gliomas, a significantly higher proportion than was previously recognized. The data also suggest that the wide range of survival times reported for patients with AA may reflect misdiagnosis, particularly of oligodendroglial tumors and pilocytic AST. The validation study on 315 additional patients was suggestive of improved categorization but limited data and no statistical analysis was provided. |
| Sharma et al7 | 43 cases of astrocytic tumors and mixed gliomas were studied evaluating the reproducibility of the Kernohan grading system compared with a computer-aided malignancy classifier | III | High inter- and intraobserver variability were observed in the Kernohan grading system, for malignant glioma the two subsequent reviewers disagreed with the initial reviewer in 8 of 15 (53%) and 11 of 15 (73%) cases. Kappa values were not calculated and could not be inferred from the data presented. Morphometric evaluation of the four histological variables with the computer assisted classifier revealed a statistically significant difference between the clusters of the measured quantitative values. Repeated grading using the computer assisted classifier and categorical assignment values of histological features resulted in complete elimination of interobserver variability. The subsequent comparison of the revised classification with survival was correlated with expected survival for malignant glioma patients but was not statistically significant. The authors conclude that the use of reproducible morphometric techniques may be of use in the grading of gliomas. This was an encouraging preliminary study on a small number of cases. |
Abbreviations: AA, anaplastic astrocytoma; AST, astrocytoma; GBM, glioblastoma; MIB–1 LI, MIB-1 labelling index
Coons et al6 reported 244 cases of glioma that were reviewed by four neuropathologists in four separate sessions to evaluate interobserver variability. Significant improvement in diagnostic concordance with each session was observed (p = 0.02). For the first session, the concordance rates were as follows: all four reviewers, 52%; any three reviewers, 60%; two reviewers, 70%. By the fourth session, this rate improved to 69%, 75%, and 80%, respectively. Kappa values were not reported and could not be calculated.
Sharma et al7 evaluated 43 cases of astrocytic tumors and mixed gliomas. They compared the reproducibility of the Kernohan grading system with a computer-aided malignancy classifier. High inter- and intraobserver variability were observed using the Kernohan grading system.8 However, morphometric evaluation of four histological variables in the classifier revealed a statistically significant difference between the clusters of the measured quantitative values. Repeated grading of histological features using a computer assisted classifier resulted in complete elimination of interobserver variability. The authors concluded that reproducible morphometric techniques might be of use in the grading of gliomas. This was an encouraging preliminary study on a small number of cases, but the limited amount of data presented precluded additional analysis.
Gaudin et al9 analyzed the diagnostic accuracy of a combination of cytopathologic and histopathologic techniques in a series of 74 patients undergoing stereotactic biopsy. Included in this study was a comparison of the crush-prep cytology technique with the frozen-section diagnosis. For those cases diagnosed as GBM, the sensitivity and specificity of the cytological technique was 0.54 and 1.0, respectively. For AA cases, the sensitivity was 0.81 and the specificity was 1.0. In a multivariate survival analysis, three factors were correlated with decreased survival: age >55 years [p < 0.001, hazard ratio = 3.58; confidence interval (CI) = 1.95, 6.57], nuclear atypia (p = 0.004, hazard ratio = 4.30; CI = 1.61, 11.46), and necrosis (p = 0.016, hazard ratio 2.14; CI = 1.15, 3.97). These authors propose that a high correlation between the cytologic crush-stain specimens and the final diagnosis could be demonstrated, and that presence of nuclear atypia and necrosis on the initial specimen could be strongly correlated with survival.
Marie et al10 described a series of 50 astrocytic gliomas and determined the interobserver variability of MIB-1 labelling index (MIB-1 LI) calculated by six pathologists using cut-off values of 2.5, 5.0, 8.0, 11.0, and 15.0%. The interobserver variability was high; the best results were with a cut-off value of 5.0% yielding a pairwise kappa value ranging from 0.52 to 0.80. The authors conclude the high level of interobserver variability suggested MIB-1 LI prognostic cutoff values might not be clinically useful for predicting outcome in individual patients with primary brain tumors. Further prospective studies are needed for investigating the prognostic usefulness of MIB-1 LI ranges that optimize interobserver agreement.
Revesz et al11 described 273 patients with gliomas and compared pathological features with survival. Data were available on 209 of 273 patients (76%). Grading, using both the Kernohan criteria and Daumas–Duport criteria,11 was compared with survival and specific pathological features examined for their contribution.11 Findings suggested both the Kernohan and Daumas–Duport classification systems significantly correlated with survival (Cox analysis, p < 0.0001), but the Kernohan did not differentiate between grades III and IV, while the Daumas–Duport did. Mitosis (p < 0.0001, x2 = 17.9), endothelial proliferation (p < 0.0001, x2 = 39.4), and necrosis (p = 0.0007, x2 = 11.5) were all significantly correlated with survival.
Martin et al12 evaluated a system of image analysis to examine 272 glioma specimens including 70 GBMs, 91 astrocytomas (ASTs), 56 pilocytic astrocytomas, and 55 oligodendrogliomas. A series of morphological properties of the nuclei and mitotic parameters were assessed, and an automated scale was created. A high degree of correlation was noted using standard grading methodology with statistically significant relationships described between numerical nuclear density, deformation of nuclei, and mitotic activity (Student’s t test, Wilcoxon’s test α = 0.05). Despite promising initial results, no data relating these findings to survival are given; hence, the sensitivity and specificity of the automated testing cannot be determined.
The study by Decaestecker et al13 proposed to incorporate computer-assisted microscopy and artificial intelligence methodology to improve the accuracy of prognostic values associated with astrocytic tumors. A series of 250 patients with glial tumors (164 GBMs, 47 AAs, and 39 astrocytomas) were included. This study demonstrated that computer-aided methodology could potentially improve conventional methods for determining prognosis based on morphologically-based classifications and suggested methodology for further refining traditional classifications into new clinically useful subgroups. The authors detected a small but significant correlation between survival and a new variable under study: the percentage of hypertriploid cell nuclei. The two factors were considered significantly correlated (r = 0.35, p < 0.0001). From the data given, the sensitivity and specificity of the test were unable to be determined.
SUMMARY
Significant variability exists in the histopathologic diagnosis of malignant glial tumors. Whether this variability always has a significant clinical impact is unclear. It appears the cytological or smear technique is similar in reliability to frozen section intraoperative studies in the diagnosis of malignant glial tumors. Methodologies to attempt to improve the diagnostic capabilities are under study. Many more will undoubtedly be available as image analysis and molecular genetic techniques evolve dramatically in the near future. For the present, the diagnosis of malignant glioma is based on histopathology and is subject to intra- and interobserver variability.
Establishing the Diagnosis
The Accuracy of Stereotactic Biopsy in Malignant Glioma
The diagnosis of a malignant glioma is often made based on the limited tissue available in a stereotactic biopsy (Fig. 3–2). To evaluate the diagnostic accuracy of stereotactic biopsy in patients with malignant glioma, we did a MEDLINE review of the medical literature using the keyword glioma and cross-referenced with biopsy, accuracy, diagnosis, variability, and pathology. As before, titles and abstracts were reviewed to remove duplicates and eliminate erroneous entries; the bibliographies of selected articles were also reviewed for additional relevant references. We selected a series of reports addressing the diagnostic accuracy of stereotactic biopsy for diagnosing malignant glioma; they are outlined below. The articles we identified focused on cases in which the intraoperative diagnosis (cytological evaluation or frozen section) could be compared with the final pathological diagnosis (open craniotomy or autopsy).
Figure 3–2 Patient prepared for image-guided stereotactic biopsy.
A number of studies have addressed the diagnostic accuracy of stereotactic biopsy in the initial management of patients suspected of harboring a malignant glioma. In the studies reviewed below, accuracy has been reported as ranging from 62 to 95%, but significant variability exists in the number of patients included and the methodology involved in these studies. The issue is one that lends itself to a well-designed study. For example, if the study includes all patients undergoing a diagnostic biopsy, who were later confirmed by craniotomy or autopsy to either have or not have a malignant glioma, the sensitivity, specificity, and positive and negative predictive value can all be calculated (Table 3-2).
Kleihues et al14 reported an early study addressing the validity of morphological diagnosis of stereotactic brain tumor biopsies. This study drew from a series of 600 patients undergoing stereotactic biopsy for presumed brain tumors. Confirmatory pathology was available by open craniotomy or autopsy in 87 cases. Cytological (smear preparations) and histological examinations of paraffin-embedded samples were compared. The biopsy diagnosis was accurate in 67 of 71 cases in which a tumor was diagnosed with a sensitivity of 0.94 and a specificity of 0.64. The authors believed inaccurate diagnoses resulted from sampling errors in nonhomogeneous tumors; that is biopsies taken from sites not representative for the entire neoplasm (tumor necrosis, infiltration zone).
Chandrasoma et al15 described a retrospective review of the diagnostic accuracy of stereotactic biopsy in 30 patients who subsequently under-went open craniotomy and compared the intraoperative diagnosis with the final diagnosis. The correlation between the stereotactic and final diagnosis was exact in 19 of 30 cases and indicated a correct clinical treatment in an additional nine cases (overall appropriate for therapy in 28 of 30 cases). There were no false-positive results for tumor and two false-negative results: One patient with glioblastoma (GBM) whose stereotactic biopsy demonstrated necrotic tissue and one patient diagnosed with granulomatous inflammation on stereotactic biopsy who was later diagnosed with a pineal germinoma. The sensitivity for the presence of tumor was 0.92 and the specificity was 1.0. The authors conclude that stereotactic technique can provide biopsy material representing the lesion with sufficient accuracy for clinical management in the majority of cases.
Feiden et al16 reported their series of 47 patients undergoing stereotactic biopsy for intracranial mass lesions with a subsequent diagnosis made at either craniotomy (38 patients) or autopsy (9 patients). The final diagnoses included low- and high-grade glial tumors as well as lymphoma, metastasis, and inflammation (aspergillosis, toxoplasmosis). In 42 cases (89%), histological results from the stereotactic biopsy and resection/autopsy were identical. The study indicated specificity for the presence of tumor of 0.87 and a specificity of 1.0. It was not possible from the data presented to calculate these values for the glial tumors alone. The authors conclude that stereotactic biopsy results in a high level of diagnostic accuracy.
Colbassani et al17 reviewed 97 patients undergoing stereotactic biopsy for diagnosis and compared frozen section with final diagnosis. In 78 cases, the frozen-section diagnosis matched the permanent diagnosis. There were five false-negative results and one false-positive result. The sensitivity was 0.94 and the specificity was 0.93. The authors conclude that intraoperative frozen sectioning is a useful and highly accurate technique to ensure appropriate tissue is obtained during a stereotactic procedure and that it results in an accurate diagnosis in a high number of cases.
Brainard et al18 conducted a retrospective review of 188 stereotactic biopsies for intracranial pathology. This study compared the results of 177 of the cases with intraoperative frozen section to the final paraffin section diagnosis. In the overall group, there was one false-positive result and 35 false-negative results. The sensitivity was 0.73 and the specificity was 0.97. The authors proposed multiple biopsies be taken until diagnostic tissue is obtained and confirmed by frozen section.
Roessler et al5 performed a retrospective analysis of cytological smears from 3541 craniotomies and 631 stereotactic biopsies. Correlation of the smear with the final diagnosis was achieved in a mean of 89.8% (range, 83 to 93.7% per year). Diagnostic accuracy increased to 95% on average (range, 91.5 to 96.7% per year) when cases of partial correlation mainly due to grading deviations were included. The accuracy of intraoperative diagnoses for glioblastoma was 95.7%. A significant reduction in diagnostic accuracy was observed in cases of oligodendroglioma (80.9%) and ependymoma (77.7%). For GBM the sensitivity was 0.97 and the specificity was 0.99. The authors note intraoperative smears are inexpensive and have high diagnostic accuracy.
Gaudin et al9 conducted a blinded review of 27 GBM and 21 AA cases comparing diagnosis based on smear cytology with final diagnosis. For glioblastoma, the cytological diagnosis had a sensitivity of 0.54 and a specificity of 1.0. For AA, the sensitivity of the cytological smear was 0.81 and the specificity was 1.0. From this small subgroup of patients, the cytological smear was effective in excluding the diagnosis of a malignant glioma (AA or GBM), but was less sensitive for the diagnosis of GBM than for AA.
| Reference | Study Design | Data Class | Conclusion | ||
|---|---|---|---|---|---|
| Jackson et al23 | Review of a consecutive series of stereotactic biopsy diagnoses with open craniotomy diagnosis in 81 patients. Review of each specimen performed by three neuropathologists. | II | Diagnoses based on biopsy vs. resection in the same patient differed in 30 (38%) of 80 cases when the biopsy slides were reviewed by three neuropathologists. Intra- and interobserver variability was not reported nor could it be calculated. For cases initially diagnosed as AA, the sensitivity was 0.67 and the specificity was 0.64. For cases initially diagnosed as GBM, the sensitivity was 0.81 and the specificity was 1.0. The study population is restricted in this study by the secondary referral. | ||
| Craniotomy diagnosis of AA | Craniotomy diagnosis of not AA | ||||
| Intraoperative diagnosis of AA | 8 | 11 | |||
| Intraoperative diagnosis of not AA | 4 | 58 | |||
| Craniotomy diagnosis of GBM | Craniotomy diagnosis of not GBM | ||||
| Intraoperative diagnosis of GBM | 36 | 0 | |||
| Intraoperative diagnosis of not GBM | 12 | 33 | |||
| This study suggests that even with expert neuropathological review the diagnosis for malignant glial tumors can frequently be inaccurate. In this group, the sensitivity of diagnosing GB was higher than for AA. | |||||
| Feiden et al16 | Review of 47 of 360 patients who underwent stereotactic biopsy followed by craniotomy (38) or autopsy (9) | II | Final diagnosis was based on permanent paraffin sections. In 42 cases (89%), the histological results in biopsy and resection/autopsy tissue were identical. There were five false-negative and no false-positive cases. The sensitivity of the initial diagnosis was 0.87 and the specificity was 1.0. The study population is restricted by those who underwent a craniotomy or the few patients who underwent craniotomy, and is non-blinded. | ||
| Craniotomy diagnosis of tumor | Craniotomy diagnosis of not tumor | ||||
| Intraoperative diagnosis of tumor | 36 | 0 | |||
| Intraoperative diagnosis of not tumor | 5 | 6 | |||
| This small study suggests that stereotactic biopsy is very specific in excluding cases of neoplasm and has a high sensitivity for establishing the diagnosis in the majority of cases. | |||||
| Chandrasoma et al15 | Study describing the pathological accuracy of brain biopsy in 30 patients with intracranial mass lesions | II | The histological diagnosis at stereotactic biopsy was appropriate for direction of clinical management in 28 of 30 patients. There were two false-negative and no false-positive cases for a sensitivity of 0.92 and a specificity of 1.0. The study population is restricted as this is only a subset reported out of an overall series of 500 biopsies. | ||
| Craniotomy diagnosis of tumor | Craniotomy diagnosis of not tumor | ||||
| Intraoperative diagnosis of tumor | 23 | 0 | |||
| Intraoperative diagnosis of not tumor | 2 | 5 | |||
| This study suggests that stereotactic biopsy can provide diagnostic accuracy that is sufficient for clinical management in the majority of cases. | |||||
| Roessler et al5 | Retrospective analysis of cytologic smears of 3541 craniotomies and 631 stereotactic biopsies | III | Correlation of the smear with the final diagnosis was achieved in a mean of 89.8% (range, 83–93.7% per year). Diagnostic accuracy increased to 95% on average (range, 91.5–96.7% per year) when cases of partial correlation, mainly due to grading deviations, were included. The accuracy of intraoperative diagnoses for GB was 95.7%. A significant reduction in diagnostic accuracy was observed in cases of oligodendroglioma (80.9%) and ependymoma (77.7%). For GBM the sensitivity was 0.97 and the specificity was 0.99. The study is retrospective and nonblinded. | ||
| Craniotomy diagnosis of GBM | Craniotomy diagnosis of not GBM | ||||
| Intraoperative cytologic diagnosis of GBM | 602 | 17 | |||
| Intraoperative cytologic diagnosis of not GBM | 14 | 4155 | |||
| The authors note that intraoperative smears are inexpensive and have high diagnostic accuracy. | |||||
| Gaudin et al9 | Blinded review of 27 GBM and 21 AA cases comparing the diagnosis based on smear cytology with the final diagnosis | II | For GBM, the cytologic diagnosis had a sensitivity of 0.54 and a specificity of 1.0. For AA, the sensitivity of the cytologic smear was 0.81 and the specificity was 1.0. Well-conducted blinded study includes analysis of cytology with final diagnosis and survival data. Numbers specifically for malignant glioma are relatively small and study not specifically designed for this subgroup so this represents a restricted number of the overall patient population under evaluation. | ||
| Final diagnosis of AA | Final diagnosis of not AA | ||||
| Cytology diagnosis of AA | 16 | 4 | |||
| Cytology diagnosis of not AA | 1 | 0 | |||
| Final diagnosis of GBM | Final diagnosis of not GBM | ||||
| Cytology diagnosis of GBM | 14 | 1 | |||
| Cytology diagnosis of not GBM | 12 | 0 | |||
| From this small subgroup of patients the cytologic smear was effective in excluding the diagnosis of a malignant glioma (AA or GBM) but was less sensitive for the diagnosis of GBM than for AA. The authors conclude that diagnosis with stereotactic biopsy using cytopathology and histopathology is accurate based on survival analysis. | |||||
| Kleihues et al14 | Review of 87 patients selected from 600 stereotactic biopsies in which biopsy was followed by craniotomy or autopsy for final diagnosis | II | The sensitivity for the diagnosis of malignant glioma was 0.94 and the specificity was 0.64. In this study there were four false-negative and five false-positive results. The study population is restricted to those patients who subsequently underwent craniotomy. | ||
| Craniotomy diagnosis of malignant glioma | Craniotomy diagnosis of not malignant glioma | ||||
| Intraoperative diagnosis of malignant glioma | 67 | 5 | |||
| Intraoperative diagnosis of not malignant glioma | 4 | 9 | |||
| The authors felt there was a significant effect of tumor heterogeneity on their results. | |||||
| Bleggi-Torres et al20 | Comparison of diagnosis based on preparation of cytologic vs. paraffin-fixed specimen in 650 cases | III | This study compares the technique of cytologic smear vs. paraffin-embedded sections prepared on the same specimen at the time of sampling. The accuracy of the smear was 97.3% with a sensitivity of 0.98, a specificity of 0.95. The authors report a positive predictive value of 0.99 and a negative predictive value of 0.90. There was one false-negative result and two false-positive results. Although these values are reported it is not possible to independently calculate them from the data provided. The authors conclude that the diagnosis based on cytologic preparation has a high correlation with paraffin-embedded specimens. | ||
| Revesz et al11 | Review of 419 stereotactic biopsy results including 273 patients with gliomas comparing the intraoperative diagnosis with the final diagnosis | II | For the diagnosis of a high-grade glial tumor by stereotactic biopsy using both cytology smear and paraffin sections, this study describes a sensitivity of 0.92 and a specificity of 0.99. There were two false-positive and 23 false-negative cases in the overall group. This study was nonblinded. | ||
| Final diagnosis of of glial tumor | Final diagnosis of of non-tumor | ||||
| Intraoperative diagnosis of glial tumor | 273 | 2 | |||
| Intraoperative diagnosis of non-tumor | 23 | 417 | |||
| Shah et al21 | Review of 183 patients with central nervous system tumors. The investigators compared to cytology vs. frozen section | II | The diagnostic accuracy of the cytology smear was 89.7% and for the frozen section was 90.4%. Overall for the diagnosis of high-grade glioma described in this study they found a sensitivity of 0.88 and a specificity of 0.99. The study is a restricted population and is nonblinded. | ||
| Final diagnosis of of high-grade glioma | Final diagnosis of of non-tumor | ||||
| Intraoperative diagnosis of high-grade glioma | 44 | 1 | |||
| Intraoperative diagnosis of not glial tumor | 6 | 132 | |||
| Brainard et al18 | Retrospective review of 188 stereotactic biopsies for intracranial pathology. This study compares the results of 177 of the cases with intraoperative frozen section with the final paraffin section diagnosis. | II | In the overall group, there was one false-positive and 35 false-negative cases. The sensitivity was 0.73 and the specificity was 0.97. This study is nonblinded and represents a retrospective review. | ||
| Final diagnosis of tumor | Final diagnosis of not tumor | ||||
| Intraoperative frozen section diagnosis of tumor | 96 | 1 | |||
| Intraoperative frozen section diagnosis of not tumor | 35 | 45 | |||
| The authors propose that multiple biopsies be taken until diagnostic tissue is obtained and confirmed by frozen section. | |||||
| Colbassani et al17 | Review of 97 patients undergoing stereotactic biopsy for diagnosis and comparison of frozen section with final diagnosis | II | Of 100 initial patients, the results with 97 are described. In 78 cases, the frozensection diagnosis matched the permanent diagnosis. There were seven falsenegative results and one false-positive result. The sensitivity was 0.94 and the specificity was 0.93. This study was nonblinded. | ||
| Final diagnosis of tumor | Final diagnosis of not tumor | ||||
| Intraoperative frozen section diagnosis of tumor | 76 | 1 | |||
| Intraoperative frozen section diagnosis of not tumor | 7 | 13 | |||
| The authors conclude that intraoperative frozen section is a useful and highly accurate technique to ensure that appropriate tissue is obtained during a stereotactic procedure and results in an accurate diagnosis in a high number of cases. | |||||
| Firlik et al22 | Retrospective review of 595 cases of stereotactic biopsy including 259 malignant glioma cases | II | For the determination of malignant glioma, there were 13 false-positive and 20 false-negative results. The sensitivity was 0.96 and the specificity was 0.75. This was a mixed patient group and nonblinded. | ||
| Final diagnostic | Final nondiagnostic | ||||
| Cytology abnormal | 523 | 13 | |||
| Cytology normal/nondiagnostic | 20 | 39 | |||
| Silverman et al19 | 31 cases of stereotactic biopsy followed by craniotomy Cytologic diagnosis compared with histologic diagnosis | II | Includes 13 cases of malignant glioma (3 AA and 10 GB). | ||
| Overall the sensitivity 0.97, specificity 1.00. Considering malignant glioma (AA as equivalent to GB) alone, the sensitivity was 0.85 and specificity was 1.0. Considering AA as distinct from GB, the sensitivity was 0.70 and the specificity was 0.90. This study represents a restricted population and is nonblinded. | |||||
| Craniotomy diagnosis of malignant glioma | Craniotomy diagnosis of not malignant glioma | ||||
| Cytology diagnosis of malignant glioma | 11 | 0 | |||
| Cytology diagnosis of not malignant glioma | 2 | 18 | |||
Abbreviations: AA, anaplastic astrocytoma; AST, astrocytoma; GBM, glioblastoma
Silverman et al19 reported 31 cases of stereotactic biopsy followed by craniotomy in which the cytological diagnosis could be compared with the final histological diagnosis. This series included 13 cases of malignant glioma (three AAs and 10 GBMs). Overall, the sensitivity for detecting a neoplasm was reported as 0.97 with a specificity of 1.00. Considering malignant glioma alone (AA as equivalent to GBM), the sensitivity was 0.85 and specificity was 1.0. Considering AA as distinct from GBM, the sensitivity was 0.70 and the specificity was 0.90.
In a study of 650 cases reported by Bleggi–Torres et al,20 the authors compared the cytological versus paraffin-fixed diagnosis prepared on the same specimen at the time of sampling. The accuracy of the smear was 97.3% with a sensitivity of 0.98 and a specificity of 0.95. The authors report a positive predictive value of 0.99 and a negative predictive value of 0.90. There was one false-negative result and two false-positive results. The authors concluded the diagnosis based on cytological preparation has a high correlation with paraffin-embedded specimens.
Revesz et al11 reviewed 419 stereotactic biopsy results, including 273 patients with gliomas, comparing the intraoperative diagnosis with the final diagnosis. For the diagnosis of a glial tumor by stereotactic biopsy using both cytology smear and a paraffin section, this study described a sensitivity of 0.92 and a specificity of 0.99. There were two false-positives and 23 false-negative cases in the overall group.
Shah et al21 studied 183 patients with central nervous system tumors. The investigators compared intraoperative cytology with frozen section. The diagnostic accuracy for the cytology smear was 89.7 and 90.4% for the frozen section. Overall, for the diagnosis of high-grade glioma described in this study, they found a sensitivity of 0.88 and a specificity of 0.99.
Firlik et al22 retrospectively reviewed 595 cases of stereotactic brain biopsy. The intraoperative cytological diagnosis correlated with the final diagnosis in 90% of cases. A diagnosis could be made in 91% of cases with sensitivity of 96% and a specificity of 75%.
Jackson et al23 retrospectively reviewed a consecutive series of 81 patients whose imaging studies suggested a glioma and who then underwent stereotactic biopsy followed by craniotomy. Outside pathologists first examined the specimens; three neuropathologists at the study institution then reinterpreted their diagnoses. Comparison of the diagnoses between the stereotactic and open specimen differed in 49% when using the diagnosis from the outside pathologist. This discrepancy was reduced to 38% when each of three neuropathologists at the study institution reviewed the biopsy slides preoperatively. No further statements on interobserver or intraobserver variability are possible from the data presented. In cases with a stereotactic biopsy diagnosis of GBM, the sensitivity was 0.81 and the specificity was 1.0; for AA, the values were 0.67 and 0.64, respectively. The authors expressed concern that stereotactic biopsy can frequently lead to an inaccurate diagnosis and should be interpreted by pathologists experienced in the grading of glial tumors. In this study, it appeared the stereotactic biopsy diagnosis of glioblastoma was associated with a higher diagnostic accuracy than AA.
SUMMARY
Stereotactic biopsy remains an option in initial patient management of suspected malignant glioma and appears to have a high likelihood of providing useful information. Heterogeneity and differentiation of reactive gliosis adjacent to either metastatic tumor or infection are potential problems that can lead to an inaccurate diagnosis. In turn, this can lead to ineffective and sometimes harmful clinical decisions and therapeutic intervention. The technical advances in neuroimaging, their effect on target selection, and the continued use of pathologists experienced in the interpretation of glial-based tumors will minimize these situations.
Determination of Prognosis
Natural History Studies
MALIGNANT GLIOMA
The prognosis of a patient with a newly diagnosed malignant glioma remains grim (Fig. 3–3). Because most patients receive some type of therapy once the diagnosis is made, information on the natural history is difficult to obtain. Despite advances in neuroimaging, surgical technique, genetic analysis, radiation therapy, and chemotherapy, improvement in survival has been minimal. According to historical controls reviewed by the American Cancer Society, the 5-year survival rate for CNS cancer (the majority being glial in origin) from 1974 to 1976 was 22%, from 1983 to 1985 was 27%, and from 1997 to 1999 was 33%. The improvement in survival was statistically significant when comparing the 1974 to 1976 data to the 1997 to 1999 data (p < 0.05).2 Despite overwhelming odds, a limited number of patients appeared to achieve a long-term survival. In evaluating the prognosis of malignant glioma, we examined the available literature on the natural history and the potential of a cure in this patient population. In our MEDLINE review, we used the keywords glioma, natural history, cure, and prognosis. Initially, we evaluated titles and abstracts to remove duplicates and eliminate erroneous entries. We reviewed bibliographies of selected articles to accumulate additional articles for consideration. Although limited in scope, several articles contained information addressing the natural history of malignant glioma and glioblastoma, and while anecdotal in nature, studies were identified that described experience with long-term survivors; we summarize them below.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






