Benjamin J. Osborne
Rod Foroozan
Mark L. Moster
In this chapter, we discuss the common neuro-ophthalmic disorders that occur in the elderly, including causes of visual loss from vascular occlusive disease [including giant cell arteritis (GCA)], optic disc swelling, orbital disease (including thyroid eye disease), disorders of ocular motility [including myasthenia gravis (MG)], and disorders of the face and eyelid.
Many neuro-ophthalmic disorders can be diagnosed solely by a complete ocular history. A thorough ocular history should include the onset and severity of visual dysfunction, the timing and progression of the visual disturbance, the presence of pain, and exacerbating and relieving factors. Specific attention should be paid to establishing whether the ocular complaint is unilateral or bilateral. A prior ocular history, including a history of strabismus, surgery, and trauma, should be sought, and any family history of ocular disease should be noted. The use of medications, both systemic and topical preparations, should be recorded in a general review of the medical history. During the examination, both eyes must be assessed, even if the patient believes that the problem is unilateral.
An important assessment of central visual function can be made by testing the best-corrected visual acuity, and each eye should be tested separately. Although most measures of visual acuity depend on subjective responses, electrophysiologic testing can be used to gain some objective measure of visual function.
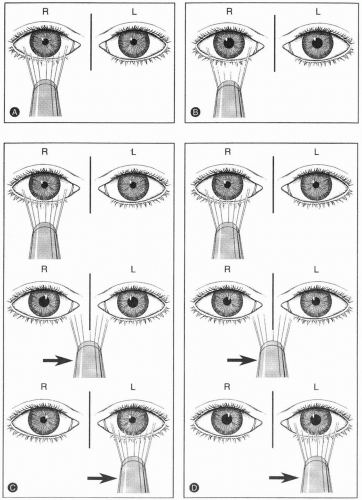
Pupillary reactivity should be assessed in the dark by instructing the patient to view a distant target, eliminating the miosis that occurs with accommodation and the near response. The response depends on the intensity of light projected and the efficiency of transmission through the afferent visual pathway (Fig. 12-1). This response is dependent on a difference between the conduction of the two afferent pupillary pathways, hence the designation relative afferent pupillary defect (RAPD), also known as a Marcus Gunn pupil (77). Patients with symmetric deficits in the afferent pupillary pathway may not have a RAPD. Instead, they show a pattern similar to that seen with a less intense light source because the conduction of the afferent pathway is less efficient.
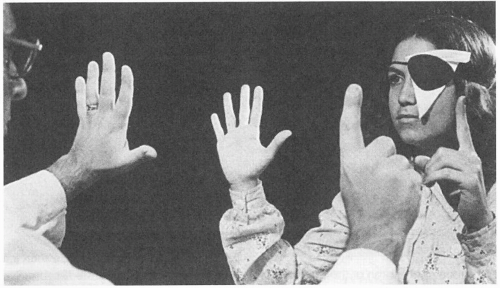
Assessment of the afferent visual system must include confrontation visual field testing. Each eye is tested separately by fully occluding the opposite eye (Fig. 12-2). The superior visual field is tested by asking the patient to count the number of fingers seen on each hand of the examiner. An impairment of either the nasal or temporal portion of the superior visual field can be confirmed by retesting each quadrant individually. Similarly, testing of the inferior visual field is performed by presenting fingers just below the level of the patient’s chin; then test the fellow eye in a similar fashion.
Electrophysiologic testing is generally not required in the evaluation of the afferent visual system but can be helpful in cases of unexplained visual loss (13). The electroretinogram (ERG) represents the sum of the response of retinal photoreceptors, and specific testing conditions can aid in differentiating the cone and rod responses. Multifocal electroretinography is a relatively new technology that measures retinal function in the central 40 to 50 degrees of the retina. It is helpful in accessing retinal disease in cases of medication toxicities (such as amiodarone, hydroxychloroquine, and ethambutol), paraneoplastic retinopathies, and retinal vascular occlusions (46). The visual evoked potential (VEP) can assess the latency and amplitude of the afferent visual response, including the visual cortex, but typically is not specific enough to determine the site of visual dysfunction.
RETINAL DISORDERS
RETINAL ARTERIAL OCCLUSIONS
Acute visual loss from retinal disease is most commonly from vascular disease. Retinal arterial occlusion, both central retinal artery occlusion (CRAO) and branch retinal artery occlusion (BRAO), causes painless loss of vision that is acute and often catastrophic. Patients with retinal artery obstruction will have visual field loss consistent with the area of occlusion, and those with CRAO will have a small RAPD. Seventy-five to 80% of patients with a CRAO will have visual acuity of counting fingers or worse (32),
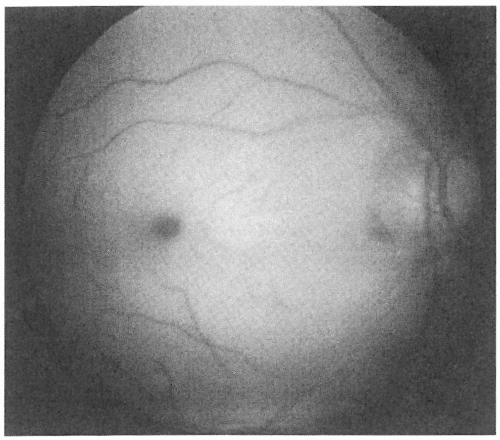
and those with BRAOs tend to have better final visual acuities (86). Funduscopy reveals retinal whitening (a marker of ischemia) in the area of obstruction (Fig. 12-3), which may enhance the darker appearance of the fovea (“cherry red spot”), as well as attenuation of the retinal arterioles and, occasionally, stagnant arterial blood flow (“box-carring”) (11). Patients with a cilioretinal artery, which has a separate blood supply than the central retinal artery, may have sparing of the macula with preserved visual acuity. The most common type of ocular arterial obstruction, CRAO, is thought to be caused by emboli, thrombosis, vasculitis, arterial spasm, and trauma. The most common visual field defect is a central scotoma followed by generalized peripheral constriction (33).
and those with BRAOs tend to have better final visual acuities (86). Funduscopy reveals retinal whitening (a marker of ischemia) in the area of obstruction (Fig. 12-3), which may enhance the darker appearance of the fovea (“cherry red spot”), as well as attenuation of the retinal arterioles and, occasionally, stagnant arterial blood flow (“box-carring”) (11). Patients with a cilioretinal artery, which has a separate blood supply than the central retinal artery, may have sparing of the macula with preserved visual acuity. The most common type of ocular arterial obstruction, CRAO, is thought to be caused by emboli, thrombosis, vasculitis, arterial spasm, and trauma. The most common visual field defect is a central scotoma followed by generalized peripheral constriction (33).
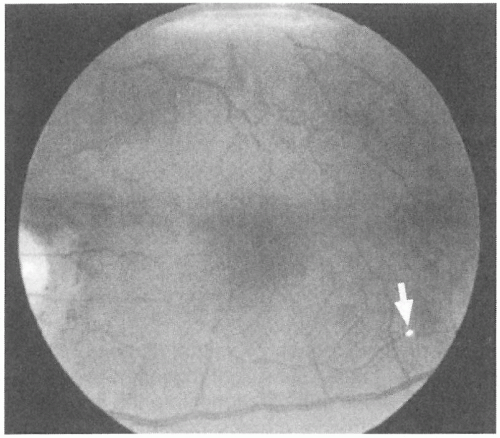
Patients with retinal vascular arterial occlusions most commonly have vasculopathic risk factors (e.g., hypertension and diabetes), and an increase in mortality from cardiac disease and stroke has been documented in patients with visible retinal arterial emboli (43,71). The evaluation of patients with retinal arterial occlusions remains controversial, but many authors suggest carotid ultrasonography and echocardiography as screening tests (73). It is important to assess elderly patients with retinal vascular occlusions for signs and symptoms of GCA because this inflammatory disorder has been estimated to account for 1% of central retinal arterial occlusions. Hence, a careful funduscopic examination should document the presence of arterial emboli (Fig. 12-4), which would largely exclude GCA as a cause of arterial occlusion.
An improvement in visual outcome has been noted in anecdotal reports of CRAO with intra-arterial thrombolytic therapy, hyperbaric oxygen, ocular massage, and anterior chamber paracentesis but only early in the course (within the first 1 to 2 hours of symptoms) (68). Despite the many treatments that have been proposed for patients presenting acutely with retinal arterial occlusions, no therapy has shown a
definitive benefit when compared with observation. The European Assessment Group for Lysis in the Eye (EAGLE) study is an ongoing prospective, randomized, multicenter study started in 2002 that is currently evaluating visual acuity 1 month after CRAO. Patients are being randomized to either a conservative treatment arm [including ocular massage and lowering intraocular pressure (IOP) with eye drops] or intra-arterial tissue-type plasminogen activator (tPA) within 20 hours of vision loss (21).
definitive benefit when compared with observation. The European Assessment Group for Lysis in the Eye (EAGLE) study is an ongoing prospective, randomized, multicenter study started in 2002 that is currently evaluating visual acuity 1 month after CRAO. Patients are being randomized to either a conservative treatment arm [including ocular massage and lowering intraocular pressure (IOP) with eye drops] or intra-arterial tissue-type plasminogen activator (tPA) within 20 hours of vision loss (21).
AMAUROSIS FUGAX
Amaurosis fugax, or a temporary loss of vision, is thought to occur from a transient occlusion of the ophthalmic circulation and is a known risk factor for acute stroke. Patients often report a dimming of the vision that lasts 10 to 20 minutes before resolution. Funduscopic findings are most commonly normal but can reveal evidence of prior retinal arterial emboli or arterial attenuation, which is an associated finding of hypertension. Elderly patients with amaurosis fugax should have carotid ultrasonography and cardiac echography in an attempt to find a source for the embolic phenomena (6).
Amaurosis fugax was one of the three symptoms evaluated in the North American Symptomatic Carotid Endarterectomy Trial (NASCET). Patients with carotid stenosis of 70% to 99% by ultrasonography who had endarterectomy had a reduced risk of subsequent stroke (9%) compared with those treated medically (26%) 2 years after treatment (58). A retrospective analysis of the NASCET trial found that patients with amaurosis fugax who had at least three of the following characteristics had a significant benefit from carotid endarterectomy: age _75 years old, history of hemispheric transient ischemic attack (TIA) or stroke, history of intermittent calf claudication, male sex, ipsilateral internal carotid artery stenosis of 80% to 94%, and no collaterals see on cerebral angiography (5). In the absence of visible emboli, patients with amaurosis fugax should be evaluated for GCA with an erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) because elderly patients with this disorder can present with transient episodes of visual loss.
RETINAL VENOUS OBSTRUCTIONS
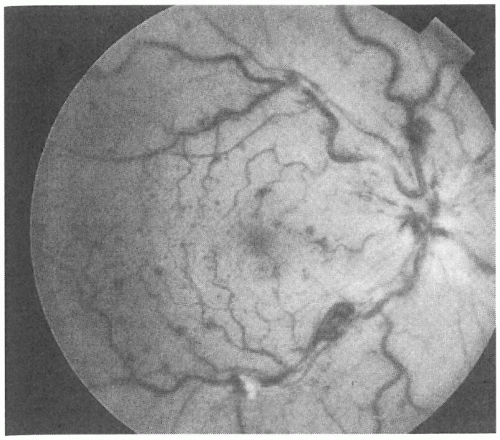
Acute painless loss of vision occurs with central retinal vein occlusion (CRVO). Risk factors for CRVO include hypertension, diabetes mellitus, and glaucoma (14). Funduscopy reveals hemorrhagic optic disc swelling, with diffuse surrounding retinal hemorrhages and dilated and tortuous retinal veins (Fig. 12-5). Exudate and subretinal fluid within the macula—the most important prognostic factors in patients presenting with vein occlusions—are chiefly responsible for the decrease in vision at presentation. Bilateral vein occlusions are atypical and should prompt a hematologic assessment for severe anemia, hypertension, thrombocytopenia, and other blood dyscrasias.
Patients with CRVO typically have visual acuities of 20/40 or worse. Ophthalmologic follow-up is essential because as many as 30% of patients with CRVO can develop neovascularization of the iris,
leading to an aggressive form of glaucoma, which requires retinal laser photocoagulation (15).
leading to an aggressive form of glaucoma, which requires retinal laser photocoagulation (15).
OCULAR ISCHEMIC SYNDROME
Similar fundus findings as seen in patients with CRVO can occur in patients with carotid occlusive disease. A 90% or greater occlusion of the carotid artery can lead to chronic ipsilateral diffuse retinal hemorrhages, mostly in the midperipheral retina, and dilated retinal veins in the ocular ischemic syndrome (OIS) (10). Patients often complain of photophobia, ocular pain, and decreased visual acuity with bright light because the hypoxia caused by carotid insufficiency is believed to cause impairment in photoreceptor metabolism, which is accentuated in bright light. Patients can have mild corneal edema and inflammation within the anterior chamber that can cause the eye to appear red from conjunctival injection. OIS is distinguished from CRVO by its chronic course, absence of optic nerve swelling, and absence of tortuous retinal veins. Patients with OIS should have close ophthalmologic follow-up because chronic hypoxia of the retina predisposes to neovascularization of the iris and can cause neovascular glaucoma (51).
ACQUIRED RETINAL DEGENERATION
Retinal dysfunction can occur in cases of cancer-associated retinopathy (CAR, or MAR for melanoma-associated retinopathy). This is a paraneoplastic syndrome caused by antibodies to retinal elements, which causes photopsia (sensation of seeing lights), nyctalopia (difficulty seeing at night), and constricted visual fields associated with retinal degeneration (42). Patients may present with sudden or subacute painless vision loss. Serum autoantibodies against retinal proteins (such as recoverin and alpha-enolase) may be seen in patients with CAR, and autoantibodies against rod bipolar cells may be seen in MAR. Patients with MAR typically already have a history of melanoma, whereas CAR usually precedes the diagnosis of the causative malignancy (usually small-cell lung cancer). No abnormality may be seen during funduscopy, especially early on in the disorder, and electrophysiologic studies with ERG may be required to document the reduced photoreceptor amplitudes. Several months later, patients may develop attenuated retinal arterioles, retinal pigment epithelial changes, and mild optic disc pallor. Intravenous methylprednisolone and plasmapheresis have been shown in some case reports to be associated with variable improvements in visual acuity, color vision, and visual fields (16).
OPTIC NEUROPATHY
The causes of optic neuropathy are extensive; however, optic nerve dysfunction, including RAPD, visual field defect, acquired color vision deficit (dyschromatopsia), and impaired contrast sensitivity, is invariably present. The onset, duration, and progression of visual symptoms will help differentiate acute from chronic causes of optic nerve dysfunction. Funduscopy in optic neuropathy often shows optic disc swelling acutely; over time, however, all causes of optic neuropathy will cause the optic nerve to appear pale. In many cases (e.g., traumatic optic neuropathy and neuropathy from compressive lesions), disc swelling may not be noted because the site of pathology lies posterior to the optic nerve head.
OPTIC DISC SWELLING (DISC EDEMA)
The presence of bilateral disc edema requires immediate attention. Associated retinal hemorrhages and cotton wool spots, including cotton wool spots of the optic nerve, are suggestive of hypertensive retinopathy. Central nervous system tumors can cause bilateral disc swelling secondary to increased intracranial pressure. Thus, patients with bilateral disc edema should have their blood pressure taken, and neuroimaging should be performed emergently to exclude a compressive lesion (see Fig. 12-6 for assessment of the elderly patient with optic disc swelling).
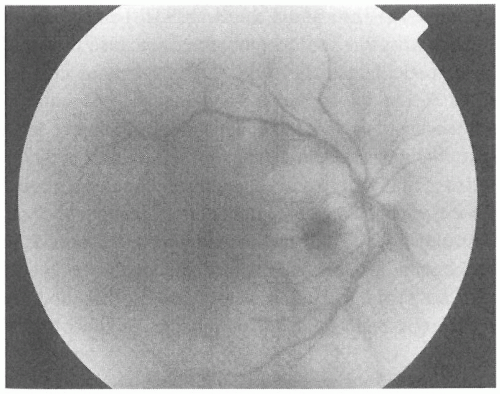
Unilateral disc swelling in elderly patients most commonly results from anterior ischemic optic neuropathy (AION). Nonarteritic ischemic optic neuropathy (NAION) occurs most commonly in vasculopathic patients in the fifth and sixth decades. Patients with NAION have an abrupt onset of painless visual loss (with visual acuities typically in the 20/60 to 20/200 range), often in the early morning hours, and an altitudinal visual field defect (36). Disc swelling, thought to be secondary to vascular insufficiency of the short posterior ciliary circulation that supplies the optic nerve head, is most often hyperemic and may be sectoral with splinter nerve fiber layer (NFL) hemorrhages. Examination of the uninvolved eye often reveals a small optic cup, suggesting a “disc at risk” for ischemia and crowding of the retinal blood vessels (19). Because NAION has only rarely been associated with embolic phenomena, carotid and cardiac ultrasonography are not indicated in the evaluation of patients with typical NAION. Some risk factors for NAION have been identified, including diabetes mellitus, hypertension, smoking, and nocturnal hypotension. In patients with NAION, the risk of infarction of the fellow optic nerve over 5 years is approximately 15% (57). The risk for ipsilateral
recurrence is quite small, at approximately 6% (30). Although some patients with NAION may have an improvement in visual acuity over the ensuing months, most will be left with a significant visual deficit. Inferior altitudinal and inferior nasal defects are the most common field cuts associated with NAION (33). No medical or surgical therapy has shown consistent benefit for patients with NAION. Some authors have suggested that aspirin may prevent infarction of the fellow eye (4), but long-term benefit has not been proven.
recurrence is quite small, at approximately 6% (30). Although some patients with NAION may have an improvement in visual acuity over the ensuing months, most will be left with a significant visual deficit. Inferior altitudinal and inferior nasal defects are the most common field cuts associated with NAION (33). No medical or surgical therapy has shown consistent benefit for patients with NAION. Some authors have suggested that aspirin may prevent infarction of the fellow eye (4), but long-term benefit has not been proven.
A number of patients using the antiarrhythmic amiodarone have been noted to have disc swelling that is virtually indistinguishable from that caused by NAION (49,56,61). However, patients in some of these cases have shown a more progressive visual deficit and bilateral involvement, which are uncommon in NAION. Amiodarone also causes crystalline deposits within the superficial cornea, in a whorl-like fashion, that are not visually disabling. Because the progression of visual loss has been halted in some patients after discontinuing this agent, patients on amiodarone with what appears to be NAION should be carefully observed for progression and fellow eye involvement (8). Patients should not discontinue amiodarone without first consulting their cardiologist (55).
Recently, there have been reports in the medical literature of an association between NAION and the use of erectile dysfunction medications, such as sildenafil, tadalafil, and vardenafil (81). It is unclear whether there is a causal relationship because some of the patients using erectile dysfunction medications have some vascular risk factors that are also associated with NAION, such as diabetes, hypertension, and hypercholesterolemia.
Patients with diabetes mellitus, often with poorly controlled blood sugar levels, may present with hyperemic disc swelling and mild visual dysfunction
(sometimes without visual complaints), which are consistent with diabetic papillopathy (65). The disc swelling and visual deficit will typically resolve weeks to months after the onset of symptoms.
(sometimes without visual complaints), which are consistent with diabetic papillopathy (65). The disc swelling and visual deficit will typically resolve weeks to months after the onset of symptoms.
AION from GCA occurs most commonly in patients in their sixth through eighth decades (mean age, 75 years), and women are affected more commonly than men. GCA is more common in whites and people from Northern Europe and is quite rare in African-Americans and Asians (63). Acute visual loss may occur after a history of brief episodes of transient visual loss. Visual acuities and visual field defects in AION caused by GCA are more commonly worse than those in patients with NAION. The disc edema of GCA is often noted to be “chalky white” in appearance because of the diffuse pallid swelling (Fig. 12-7). A history of headache, scalp tenderness, jaw claudication, fever, malaise, decreased appetite, weight loss, and symptoms of polymyalgia rheumatica should be sought (23). Other causes of vision loss from GCA include branch and CRAO and posterior ischemic optic neuropathy (PION). In addition to optic neuropathy from AION, other cranial nerves can be affected in GCA, and diplopia, facial nerve palsy, and facial pain have been noted.
Patients suspected of having GCA should have ESR and CRP drawn and be placed on corticosteroids without delay to prevent visual loss in the fellow eye; visual loss in the affected eye is typically irreversible. Although most patients with GCA will have an elevated ESR (estimates of up to 24% of biopsy-proved cases may not), those patients suspected of having GCA should have biopsy of the superficial temporal artery to confirm the diagnosis. An elevated CRP level may be helpful in suspicious cases with a normal ESR, and bilateral biopsy should be considered in cases with a high clinical suspicion because skip lesions can lead to a false-negative biopsy result. The variables with the highest positive predictive value for a positive temporal artery biopsy are jaw claudication, scalp tenderness, new headache, and vision loss (85). Most patients require high doses of corticosteroids (prednisone, 60 to 100 mg) for at least 6 months to 1 year. Attempts to taper steroid therapy should be very gradual, with close monitoring of visual function, ESR, and CRP.
Despite the differences outlined above, the disc swelling from NAION can be difficult to distinguish from that caused by GCA, and ESR and CRP studies are warranted in all patients _55 years with optic disc edema thought to be caused by ischemic optic neuropathy.
Optic nerve tumors (e.g., malignant optic gliomas and meningiomas) can cause optic disc swelling; however, more commonly, optic atrophy is present with these lesions. Patients may present with findings suggestive of an acute optic neuropathy without evidence of optic disc swelling, the so-called “posterior ischemic optic neuropathy” (PION) thought to be caused by ischemia of the retrobulbar circulation of the optic nerve (35). The average age of patients with PION is 52 years old (12). Optic neuropathy from PION has been noted after acute ischemic states, such as after cardiac bypass or spine surgery, and in patients with GCA. PION is a rare complication of spinal surgery and has been associated with spinal surgeries with blood loss of more than 1 L and anesthesia duration over 6 hours. Usually the vision loss is bilateral and severe (47). Patients suspected of having PION, without a history of an acute ischemic event, should have an ESR and CRP drawn to rule out GCA (31). Electrophysiologic testing showing a normal ERG and absent VEP is suggestive of PION and can help confirm the diagnosis.
A rare cause of vision loss in patients with cancer is paraneoplastic optic neuropathy. The vision loss is typically acute to subacute and painless. Patients may have associated neurologic signs such as chorea, ataxia, peripheral neuropathy, and dementia. Funduscopy shows bilateral optic disk swelling. Autoantibodies against collapsing response-mediating protein-5 (CRMP-5) are commonly found and are predominantly associated with small cell lung cancer (16).
OPTIC ATROPHY
GLAUCOMA
Glaucomatous optic atrophy is characterized by optic nerve cupping and elevated intraocular pressure (IOP)
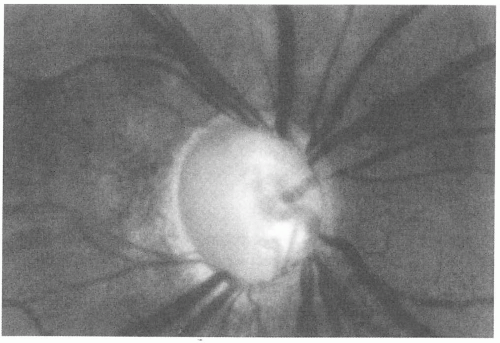
in the presence of visual field defects. It is estimated that 1% to 2% of the population has glaucoma, and 50% are thought to be unaware of the diagnosis (62). Hence, a screening fundus examination is paramount to the diagnosis. Elevated IOP, the only known modifiable risk factor, is not present in all cases of glaucomatous optic neuropathy. Typically, involvement is bilateral, but it can be asymmetric. Older age is an important risk factor for glaucomatous optic atrophy, and a family history of glaucoma is frequently present in first-degree relatives. Optic atrophy in glaucoma is typically associated with disc pallor within the area of cupping (Fig. 12-8), but pallor that appears to be out of proportion to the degree of cupping should prompt further investigation (2). Visual loss in glaucoma is most often progressive, with loss of the peripheral visual field occurring before loss of central fixation. It is often difficult to make a diagnosis solely on one encounter because IOP can fluctuate, and close follow-up with quantitative visual field testing is important.
in the presence of visual field defects. It is estimated that 1% to 2% of the population has glaucoma, and 50% are thought to be unaware of the diagnosis (62). Hence, a screening fundus examination is paramount to the diagnosis. Elevated IOP, the only known modifiable risk factor, is not present in all cases of glaucomatous optic neuropathy. Typically, involvement is bilateral, but it can be asymmetric. Older age is an important risk factor for glaucomatous optic atrophy, and a family history of glaucoma is frequently present in first-degree relatives. Optic atrophy in glaucoma is typically associated with disc pallor within the area of cupping (Fig. 12-8), but pallor that appears to be out of proportion to the degree of cupping should prompt further investigation (2). Visual loss in glaucoma is most often progressive, with loss of the peripheral visual field occurring before loss of central fixation. It is often difficult to make a diagnosis solely on one encounter because IOP can fluctuate, and close follow-up with quantitative visual field testing is important.
Although open angle glaucoma accounts for more than 90% of all patients with glaucoma in the United States, angle closure glaucoma can result in diffuse conjunctival injection, ocular pain, nausea, and vomiting from an extreme rise in IOP (2). However, some cases of chronic angle closure may not manifest these more acute findings and will require ophthalmologic examination to reveal the cause of visual dysfunction.
OPTIC NERVE MENINGIOMAS
Meningiomas of the sphenoid wing and, less commonly, the optic nerve cause progressive visual loss, optic disc pallor, and sometimes optic disc venous collaterals or “shunt vessels” (20). Meningiomas are most commonly found in middle-aged and elderly women, and an external examination may reveal a mass within the temporal fossa. Although surgery is often recommended for external compression, radiation is very helpful, particularly for optic nerve sheath meningiomas. More than half of patients treated with stereotactic fractionated radiotherapy will show some improvement in their vision within 3 months (50,53).
OTHER CAUSES OF OPTIC ATROPHY
The finding of unexplained optic atrophy requires investigation. A family history of visual loss may suggest a hereditary optic neuropathy, and a remote history of trauma may suggest traumatic optic neuropathy. Patients with a history of alcohol abuse and smokers are at risk for developing a form of toxic optic neuropathy characterized by visual field defects that involve fixation and the blind spot (cecocentral scotomas) (66). In addition, a dietary history should be obtained because many of these patients are deficient in the essential B vitamins. A long list of medications is associated with optic neuropathy, which can first present acutely with disc swelling and show progressive disc pallor.
It is important to assess potentially treatable conditions that can cause unexplained optic atrophy; hence, neuroimaging with attention to the optic nerves is recommended. In addition, testing for syphilis, sarcoidosis, Lyme disease, and nutritional deficiency (vitamin B12 and folate) should be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree