Neuroanatomy
R. C. A. Pearson†
† Dr. Pearson died while this new edition was being prepared. The editors pay tribute to his scientific achievements and to his contributions to this book.
Introduction
The symptoms, signs, and syndromes of psychiatry, whether organic or biological psychiatric disease or not, in the main reflect alterations in functions which reside in the cerebral cortex, including the limbic lobe, and those structures and pathways closely related to the cortex. These cortical manifestations of psychiatric disease include alterations in thought, language, perception, mood, memory, motivation, personality, behaviour, and intellect. Therefore, this brief account of brain structures and pathways that are important in psychiatry will concentrate on the cerebral cortex and related structures and pathways. Readers who require a fuller account of central nervous system anatomy are referred to the many standard texts, which give a more complete coverage of the subject.
Broadly speaking, neuroanatomy can be subdivided into two parts—the topographical organization of the brain and spinal cord, and the anatomical connections forming functional pathways in the central nervous system. The former is of vital importance clinically, since pathologies rarely respect the boundaries of functional systems, and knowledge of the spatial relationships of different brain structures is increasingly useful as modern imaging methods more accurately visualize detailed brain structure in vivo. However, it is the second subdivision of the subject which makes the greater contribution to understanding the biological basis of psychiatric disease, and it is this that will be at the centre of the present account.
The structure and organization of the cerebral cortex
The lobes of the cerebral cortex
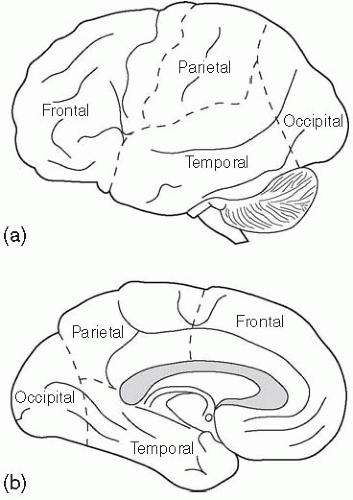
A variable pattern of fissures (sulci) and folds (gyri), many of which have specific names, extensively groove the surface of the cerebral hemisphere. A few are relatively constant and are used to subdivide the cerebral hemisphere into lobes, named for the bones of the skull which they underlie (Fig. 2.3.1.1 ).
The deep lateral sulcus, also called the Sylvian fissure, extends from the uncus, anteriorly and medially, to the parietal lobe, posteriorly and medially. It has a short stem, and anterior, ascending, and posterior rami. The anterior and ascending rami embrace the pars triangularis of the frontal lobe, which houses Broca’s motor speech area. The much longer posterior ramus is used in defining the lobes of the hemisphere. The central sulcus is prominent approximately midway along the anteroposterior extent of the
lateral surface of the hemisphere and, most commonly, extends over the medial margin, where its inferomedial tip is embraced by the U-shaped paracentral lobule. On the lateral surface, it passes from the medial margin, forwards and laterally, to reach the lateral sulcus. The line of the central sulcus closely approximates the line of the coronal suture of the adult skull, i.e. the junction between the frontal and parietal bones; consequently, the sulcus separates the frontal and parietal lobes. The demarcation of the occipital lobe is the parieto-occipital sulcus dorsally and medially, and the preoccipital sulcus ventrally and laterally, with an imaginary line connecting the two and intersecting the posterior tip of the lateral sulcus. The temporal lobe lies anterior to this line and inferior (ventral) to the lateral sulcus. The deep lateral sulcus broadens out at its fundus, with an area of cortex forming the extensive floor of the sulcus, particularly in its anterior two-thirds. This cortex is the insula, which does not form part of any of the lobes mentioned above. The insula is surrounded by the circular sulcus, and is overhung by the frontal and parietal opercula superiorly, and the temporal operculum inferiorly (ventrally). The anatomical borders of the lobes of the cerebral cortex, and other sulcal and gyral landmarks, are only loosely paralleled by functional boundaries. However, lobar terminology is so firmly embedded in clinical and non-clinical neuroscience that consideration of their anatomical features is essential.
lateral surface of the hemisphere and, most commonly, extends over the medial margin, where its inferomedial tip is embraced by the U-shaped paracentral lobule. On the lateral surface, it passes from the medial margin, forwards and laterally, to reach the lateral sulcus. The line of the central sulcus closely approximates the line of the coronal suture of the adult skull, i.e. the junction between the frontal and parietal bones; consequently, the sulcus separates the frontal and parietal lobes. The demarcation of the occipital lobe is the parieto-occipital sulcus dorsally and medially, and the preoccipital sulcus ventrally and laterally, with an imaginary line connecting the two and intersecting the posterior tip of the lateral sulcus. The temporal lobe lies anterior to this line and inferior (ventral) to the lateral sulcus. The deep lateral sulcus broadens out at its fundus, with an area of cortex forming the extensive floor of the sulcus, particularly in its anterior two-thirds. This cortex is the insula, which does not form part of any of the lobes mentioned above. The insula is surrounded by the circular sulcus, and is overhung by the frontal and parietal opercula superiorly, and the temporal operculum inferiorly (ventrally). The anatomical borders of the lobes of the cerebral cortex, and other sulcal and gyral landmarks, are only loosely paralleled by functional boundaries. However, lobar terminology is so firmly embedded in clinical and non-clinical neuroscience that consideration of their anatomical features is essential.
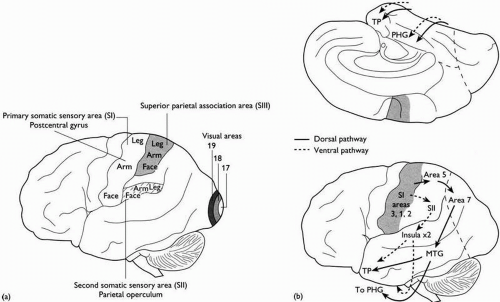
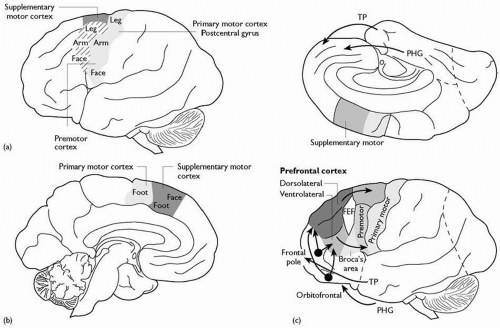 Fig. 2.3.1.2 Motor areas of the frontal lobe: TP, temporal pole; PHG, parahippocampal gyrus; FEF, frontal eyefields. |
(a) The frontal lobe (Fig. 2.3.1.2)
The precentral gyrus, immediately in front of the central sulcus and continuing onto the medial surface, contains the primary motor cortex. The precentral sulcus usually defines the anterior boundary, and in front of this lies the premotor cortex. The inferior
margin of the sulcus runs into the pars triangularis, which includes Broca’s motor speech area. On the medial margin and surface, the cortex includes the supplementary motor area. The lateral prefrontal cortex, in front of these motor and associated areas, is usually grooved by two major horizontal sulci, defining the superior, middle, and inferior frontal gyri. The cortex on the medial surface of the frontal lobe anterior to the prefrontal gyrus forms the medial prefrontal cortex. The concave inferior surface of the frontal lobe, overlying the bony orbit in vivo, is the orbitofrontal cortex.
margin of the sulcus runs into the pars triangularis, which includes Broca’s motor speech area. On the medial margin and surface, the cortex includes the supplementary motor area. The lateral prefrontal cortex, in front of these motor and associated areas, is usually grooved by two major horizontal sulci, defining the superior, middle, and inferior frontal gyri. The cortex on the medial surface of the frontal lobe anterior to the prefrontal gyrus forms the medial prefrontal cortex. The concave inferior surface of the frontal lobe, overlying the bony orbit in vivo, is the orbitofrontal cortex.
(b) The parietal lobe (Fig. 2.3.1.3)
Behind the central sulcus, the primary somatic sensory cortex (SI), extending onto the medial surface to occupy the posterior part of the paracentral lobule, where the sacral spinal segments are represented, occupies the postcentral gyrus. The postcentral sulcus limits the postcentral gyrus posteriorly. Behind this, the sulcal pattern is variable, but one or more horizontal intraparietal sulci divide the lobe into superior and inferior parts. The second somatic sensory cortex (SII) is located in the parietal operculum, close behind the inferolateral tip of the central sulcus. Specific sulcal patterns in the transition region between the parietal lobe and the occipital and temporal lobes (the supramarginal and angular gyri) are important landmarks for the detailed localization of language functions.
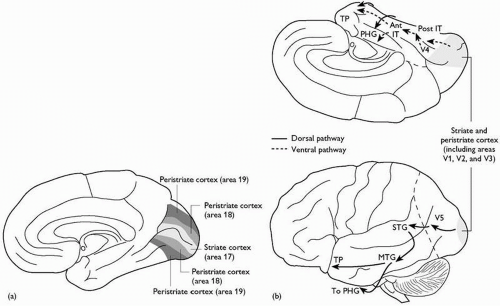
(c) The occipital lobe (Fig. 2.3.1.4)
The occipital lobe is predominantly involved in vision and visual perception. The medial surface is grooved by the deep horizontal calcarine sulcus, which typically reaches the posterior pole of the hemisphere. Within its walls is the primary visual cortex. This area (area 17 of Brodmann) is often called the striate cortex; in the freshly sliced brain, a thin band of white matter, the stria of Gennari, is clearly visible running in the centre of the cortical grey ribbon. The extent of this stria precisely demarcates the primary visual cortex. Surrounding areas of the medial and lateral surface, the prestriate and peristriate cortex, contain some of the numerous separate visual association areas.
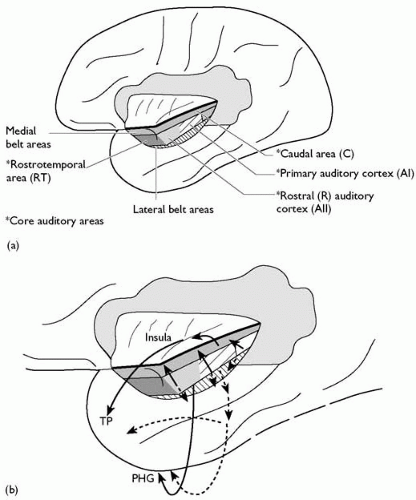
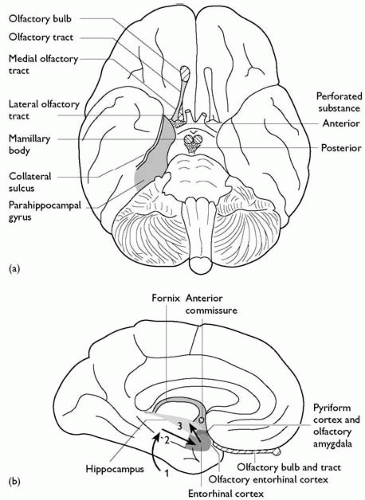
(d) The temporal lobe (Figs 2.3.1.5 and 2.3.1.6)
Two horizontal sulci, the superior and inferior temporal sulci, divide the lateral surface of the temporal lobe into the superior, middle, and inferior gyri. The latter, extending onto the inferior surface, is also known as the inferotemporal cortex. On the medial surface, the collateral sulcus runs from close to the temporal pole to the calcarine sulcus posteriorly. Medial to this is the parahippocampal gyrus. Anteriorly, this curves dorsally and caudally to form the hook-shaped uncus. The entorhinal cortex occupies approximately the anterior third of the parahippocampal gyrus. Lateral to this, in the walls of the rhinal sulcus, lies the perirhinal cortex. The uncus closely overlies the amygdala; the primary olfactory cortex, the piriform cortex, lies immediately in front. The choroid fissure limits the parahippocampal gyrus medially. Passing into the floor of the lateral ventricle, the subicular areas of cortex lead to the hippocampus proper. A detailed consideration of the anatomy of the hippocampus is given below.
The cortex of the temporal operculum contains Heschl’s gyri, within which lies the primary auditory cortex. Diverse auditory association areas surround this, extending into the superior temporal gyrus. The functions of the cortex of the middle temporal gyrus are uncertain, but include complex visual, auditory, and somatic sensory association areas. The inferotemporal cortex is largely concerned with visual perception and cognition. The anatomical pathways that underlie these functions are considered below.
(e) The insula
The anterior margin of the insula, where the cortex becomes continuous with the anterior perforated substance, is known as the limen insulae. Above and below, where the insular cortex rolls round onto the opercula, lies the circular sulcus, the superior and inferior rami of which fuse posterosuperiorly to form the apex of the insula. Several variable sulci mark the insula, but little is known of the functional subdivisions of this cortex; gustatory, somatic sensory, and auditory areas have been described.
The structure of the neocortex
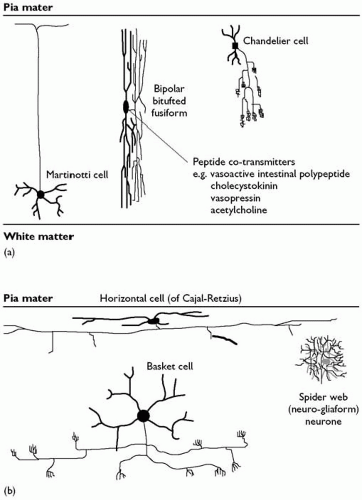
The neocortical grey matter is usually described as having six layers (Plate 1(a)). Wide variation in the nature of this microscopic lamination underlies the subdivision of neocortex into a multiplicity of (usually numbered) areas. At its simplest, two types of neurones make up the grey matter—pyramidal and non-pyramidal (or granular) cells. An apparent predominance of one or other type gives the extremes of granular and agranular cortex, equating with sensory areas (granular) and the motor cortex (agranular). In fact, the proportion of different cell types is constant in all areas. Indeed, with the single exception of the primary visual cortex, the numbers of neurones under a fixed surface area is also constant in all cortical areas. Variations in the size of pyramidal cells in particular lead to an apparent change in proportions. These variations probably reflect differences in the axonal volume of individual pyramidal cells, reflecting the distance and volume of projection fibres from a cortical area.
Pyramidal cells have a single main apical dendrite ascending towards the pial surface, and several horizontally spreading basal dendrites. All dendrites bear dendritic spines, which receive synapses (Plate 1(b)). All pyramidal cells use excitatory amino acids as neurotransmitters and have axons which enter the subcortical white matter; hence they are all projection neurones. They constitute approximately 60 per cent of all the neurones in the cortex. A second spiny neuronal type, the spiny stellate cells, is the next most numerous. These also use an excitatory amino acid, most probably glutamate, as their neurotransmitter. Unlike pyramidal cells, however, their axons remain confined to the cortical grey matter; they are interneurones, accounting for a further 25 per cent of cortical neurones. All the other neurones are inhibitory interneurones, using γ-aminobutyric acid (GABA) as their major neurotransmitter. Many also contain one or more neuropeptides, and their content of specific calcium-binding proteins varies. They have a wide range of axonal and dendritic forms, and have been multiply classified in the past. Broadly speaking, they can be grouped into those with horizontal axonal arborizations, those whose axons ramify at right angles to the pial surface, i.e. through the depth of the cortex, and those with radial axons (Fig. 2.3.1.7).
The structure of the allocortex
The allocortex comprises a number of different areas, all with very different structures. They are either limbic or olfactory (or both) and are found predominantly in the medial temporal lobe. The largest of these regions is the hippocampus. Essentially, this includes the three-layered cortex of the hippocampus, together with the transitional areas between it and neocortex, which are variably said to have three, four, five, or six laminae. The hippocampal formation comprises the dentate gyrus, Ammon’s horn (CA fields), and the subiculum. Both the dentate gyrus and the CA fields have a prominent single layer of neurones, with an overlying molecular layer and a subjacent polymorphic layer. In the dentate gyrus, the cells are granule cells, whereas in the CA fields they are predominantly large pyramidal cells—the stratum pyramidale. Both cell types are excitatory and are projection neurones. Scattered populations of GABA-ergic inhibitory interneurones are found immediately subjacent to the main cellular laminae and in the molecular layers. The CA fields are numbered 1, 2, and 3, from the subiculum to the dentate gyrus. The subiculum is the zone of transition between the three-layered hippocampus proper, and the entorhinal cortex and cortex of the parahippocampal gyrus laterally. It is sometimes further subdivided into subzones including the presubiculum, between the lateral cortex and the subiculum, and the prosubiculum, between the subiculum proper and CA1 of the hippocampus. The histological appearance of the hippocampus and adjacent areas are shown in Fig. 2.3.1.8, and their connections are considered in detail below.
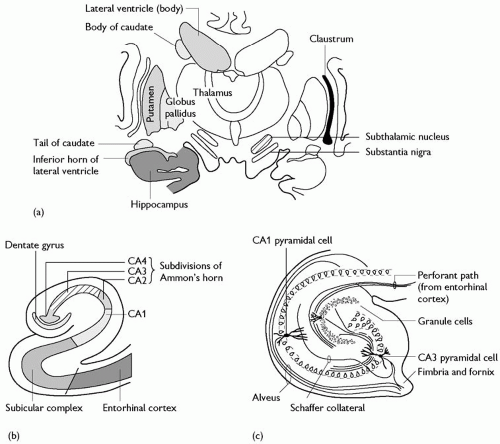 Fig. 2.3.1.8 (a) Hippocampal formation in the temporal lobe; (b) subdivisions of the hippocampal formation; (c) organization of the hippocampus. |
Table 2.3.1.1 General connections of the cerebral cortex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The general pattern of connectivity of the cortex
All cortical areas share a broadly similar pattern of connections. What differs is the relative quantity of connections in each category, as well as the precise detail of origin and termination. The broad categories of connections which all cortical areas share are most easily seen in a table (Table 2.3.1.1). These will be dealt in detail in subsequent sections.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree