Neurocognitive Disorders
 21.1 Introduction and Overview
21.1 Introduction and Overview
Advances in molecular biology diagnostic techniques and medication management have significantly improved the ability to recognize and treat cognitive disorders. Cognition includes memory, language, orientation, judgment, conducting interpersonal relationships, performing actions (praxis), and problem solving. Cognitive disorders reflect disruption in one or more of these domains and are frequently complicated by behavioral symptoms. Cognitive disorders exemplify the complex interface among neurology, medicine, and psychiatry in that medical or neurological conditions often lead to cognitive disorders that, in turn, are associated with behavioral symptoms. It can be argued that of all psychiatric conditions, cognitive disorders best demonstrate how biological insults result in behavioral symptomatology. The clinician must carefully assess the history and context of the presentation of these disorders before arriving at a diagnosis and treatment plan.
This century-old distinction between organic and functional disorders is outdated and has been deleted from the nomenclature. Every psychiatric disorder has an organic (i.e., biological or chemical) component. Because of this reassessment, the concept of functional disorders has been determined to be misleading, and the term functional and its historical opposite, organic, are no longer used in the current Diagnostic and Statistical Manual of Mental Disorders (DSM) nomenclature. A further indication that the dichotomy is no longer valid is the revival of the term neuropsychiatry, which emphasizes the somatic substructure on which mental operations and emotions are based; it is concerned with the psychopathological accompaniments of brain dysfunction as observed in seizure disorders, for example. Neuropsychiatry focuses on the psychiatric aspects of neurological disorders and the role of brain dysfunction in psychiatric disorders.
Cognitive disorders tend to defy Occam’s razor, challenging clinicians and nosologists with multiplicity, comorbidity, and unclear boundaries. These concerns are most true in elderly adults, the demographic group most at risk for cognitive disorders. Dementias of late life are particularly problematic in this regard. Existing, although often unrecognized, dementia is a major risk factor for superimposed delirium. Moreover, certain dementias, such as dementia with Lewy bodies or late stages of Alzheimer’s disease, may have chronic clinical presentations virtually indistinguishable from delirium except for temporal onset and the lack of an identifiable acute source. Similarly, the course of nearly all subjects developing a progressive dementia is complicated by the onset of one or more distinct behavioral syndromes, including anxiety, depression, sleep problems, psychosis, and aggression. These symptoms can be as distressing and disabling as the primary cognitive disorder. Some of these behavioral syndromes, such as psychosis, may themselves result from independent underlying biologies and may be additive with the primary neurodegenerative process.
The boundaries between types of dementia and between dementia and normal aging can be similarly diffuse. Neuropathologic studies of both clinical and population samples have revealed a surprising truth. The most common neuropathologic presentation associated with dementia reveal mixtures of Alzheimer’s disease, vascular, and Lewy body pathologies. Pure syndromes are relatively less common, although often the dementia is ascribed to one of the coexisting pathologies. Strategies regarding how to understand or reconcile multiple pathologies in the clinic are needed, although they lag behind.
DEFINITION
Delirium
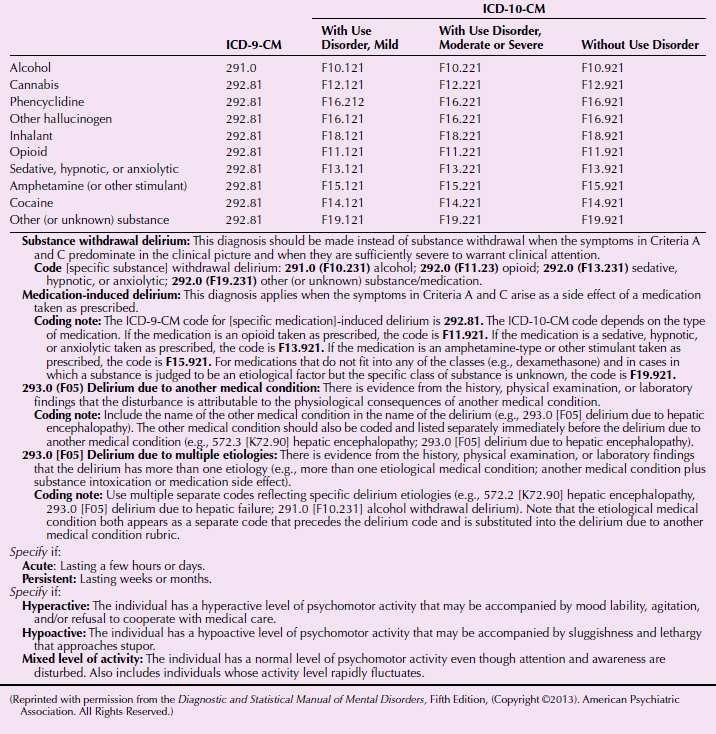
Delirium is marked by short-term confusion and changes in cognition. There are four subcategories based on several causes: (1) general medical condition (e.g., infection), (2) substance induced (e.g., cocaine, opioids, phencyclidine [PCP]), (3) multiple causes (e.g., head trauma and kidney disease), and (4) other or multiple etiologies (e.g., sleep deprivation, mediation). Delirium is discussed in Section 21.2.
Dementia (Major Neurocogntive Disorder)
Dementia, also referred to as major neurocognitive disorder in the fifth edition of DSM (DSM-5), is marked by severe impairment in memory, judgment, orientation, and cognition. The subcategories are (1) dementia of the Alzheimer’s type, which usually occurs in persons older than 65 years of age and is manifested by progressive intellectual disorientation and dementia, delusions, or depression; (2) vascular dementia, caused by vessel thrombosis or hemorrhage; (3) human immunodeficiency virus (HIV) disease; (4) head trauma; (5) Pick’s disease or frontotemporal lobar degeneration; (6) Prion disease such as Creutzfeldt-Jakob disease, which is caused by a slow-growing transmittable virus); (7) substance induced, caused by toxin or medication (e.g., gasoline fumes, atropine); (8) multiple etiologies; and (9) not specified (if cause is unknown).
In DSM-5, a less severe form of dementia called mild neurocognitive disorder is listed. Dementia is discussed in Section 21.3.
Amnestic Disorder
Amnestic disorders are classified in DSM-5 as major neurocognitive disorders caused by other medical conditions. They are marked primarily by memory impairment in addition to other cognitive symptoms. They may be caused by (1) medical conditions (hypoxia), (2) toxins or medications (e.g., marijuana, diazepam), and (3) unknown causes. These disorders are discussed in Section 21.4.
CLINICAL EVALUATION
During the history taking, the clinician seeks to elicit the development of the illness. Subtle cognitive disorders, fluctuating symptoms, and progressing disease processes may be tracked effectively. The clinician should obtain a detailed rendition of changes in the patient’s daily routine involving such factors as self-care, job responsibilities, and work habits; meal preparation; shopping and personal support; interactions with friends; hobbies and sports; reading interests; religious, social, and recreational activities; and ability to maintain personal finances. Understanding the past life of each patient provides an invaluable source of baseline data regarding changes in function, such as attention and concentration, intellectual abilities, personality, motor skills, and mood and perception. The examiner seeks to find the particular pursuits that the patient considers most important, or central, to his or her lifestyle and attempts to discern how those pursuits have been affected by the emerging clinical condition. Such a method provides the opportunity to appraise both the impact of the illness and the patient-specific baseline for monitoring the effects of future therapies.
Mental Status Examination
After taking a thorough history, the clinician’s primary tool is the assessment of the patient’s mental status. As with the physical examination, the mental status examination is a means of surveying functions and abilities to allow a definition of personal strengths and weakness. It is a repeatable, structured assessment of symptoms and signs that promotes effective communication among clinicians. It also establishes the basis for future comparison, essential for documenting therapeutic effectiveness, and it allows comparisons between different patients, with a generalization of findings from one patient to another. Table 21.1-1 lists the components of a comprehensive neuropsychiatric mental status examination.
 Table 21.1-1
Table 21.1-1
Neuropsychiatric Mental Status Examination

Cognition
When testing cognitive functions, the clinician should evaluate memory; visuospatial and constructional abilities; and reading, writing, and mathematical abilities. Assessment of abstraction ability is also valuable, although a patient’s performance on tasks such as proverb interpretation may be a useful bedside projective test in some patients, the specific interpretation may result from a variety of factors, such as poor education, low intelligence, and failure to understand the concept of proverbs, as well as from a broad array of primary and secondary psychopathological disturbances.
PATHOLOGY AND LABORATORY EXAMINATION
As with all medical tests, psychiatric evaluations such as the mental status examination must be interpreted in the overall context of thorough clinical and laboratory assessment. Psychiatric and neuropsychiatric patients require careful physical examination, especially when issues exist that involve etiologically related or comorbid medical conditions. When consulting internists and other medical specialists, the clinician must ask specific questions to focus the differential diagnostic process and use the consultation most effectively. In particular, most systemic medical or primary cerebral diseases that lead to psychopathological disturbances also manifest with a variety of peripheral or central abnormalities.
A screening laboratory evaluation is sought initially and may be followed by a variety of ancillary tests to increase the diagnostic specificity. Table 21.1-2 lists such procedures, some of which are described below.
 Table 21.1-2
Table 21.1-2
Screening Laboratory Tests

ELECTROENCEPHALOGRAPHY
Electroencephalography (EEG) is an easily accessible, noninvasive test of brain dysfunction that has high sensitivity for many disorders but relatively low specificity. Beyond its recognized uses in epilepsy, EEG’s greatest utility is in detecting altered electrical rhythms associated with mild delirium, space-occupying lesions, and continuing complex partial seizures (in which the patient remains conscious, although behaviorally impaired). EEG is also sensitive to metabolic and toxic states, often showing a diffuse slowing of brain activity. The EEG is discussed in Section 3.4, Electrophysiology.
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
Computed tomography (CT) and magnetic resonance imaging (MRI) have proved to be powerful neuropsychiatric research tools. Recent developments in MRI allow the direct measurement of structures such as the thalamus, basal ganglia, hippocampus, and amygdala, as well as temporal and apical areas of the brain and the structures of the posterior fossa. MRI has largely replaced CT as the most utilitarian and cost-effective method of imaging in neuropsychiatry. Patients with acute cerebral hemorrhages or hematomas must continue to be assessed using CT, but these patients present infrequently in psychiatric settings. MRI better discriminates the interface between gray and white matter and is useful in detecting a variety of white matter lesions in the periventricular and subcortical regions. The pathophysiological significance of such findings remains to be defined. White matter abnormalities are detected in younger patients with multiple sclerosis or human immunodeficiency virus (HIV) infection and in older patients with hypertension, vascular dementia, or dementia of the Alzheimer’s type. The prevalence of these abnormalities is also increased in healthy, aging individuals who have no defined disease process. As with CT, the greatest utility of MRI in the evaluation of patients with dementia arises from what it may exclude (tumors, vascular disease) rather than what it can demonstrate specifically.
BRAIN BIOPSY
Brain needle biopsy is used to diagnose a variety of disorders: Alzheimers disease, autoimmune encephalopaties, and tumors. It is conducted stereotactically and indicated when no other investigative techniques such as MRI or lumbar puncture have been sufficient to make a diagnosis. The procedure is not without risk in that seizures may occur if scar tissue forms at the biopsy site.
NEUROPSYCHOLOGICAL TESTING
Neuropsychological testing provides a standardized, quantitative, reproducible evaluation of a patient’s cognitive abilities. Such procedures may be useful for initial evaluation and periodic assessment. Tests are available that assess abilities across the broad array of cognitive domains, and many offer comparative normative groups or adjusted scores based on normative samples. The clinician seeking neuropsychological consultation should understand enough about the strengths and weaknesses of selected procedures to benefit fully from the results obtained.
REFERENCES
Balzer D. Neurocognitive disorders in DSM-5. Am J Psych. 2013;170:585.
Blanc-Lapierre A, Bouvier G, Gruber A, Leffondré K, Lebailly P, Fabrigoule C, Baldi I. Cognitive disorders and occupational exposure to organophosphates: Results from the PHYTONER Study. Am J Epidemiol. 2013;177:1086.
Bugnicourt J-M, Godefroy O, Chillon J-M, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353.
Bugnicourt J-M, Guegan-Massardier E, Roussel M, Martinaud O, Canaple S, Triquenot-Bagan A, Wallon D, Lamy C, Leclercq C, Hannequin D, Godefroy O. Cognitive impairment after cerebral venous thrombosis: A two-center study. J Neurol. 2013;260:1324.
Fields J, Dumaop W, Langford TD, Rockenstein E, Masliah E. Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J Neuroimmune Pharmacol. 2014;9(2):102–116.
Jack CR Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665.
Launer LJ. Epidemiologic insight into blood pressure and cognitive disorders. In: Yaffe K, ed. Chronic Medical Disease and Cognitive Aging: Toward a Healthy Body and Brain. New York: Oxford University Press; 2013:1.
Mayeux R, Reitz C, Brickman AM, Haan MN, Manly JJ, Glymour MM, Weiss CC, Yaffe K, Middleton L, Hendrie HC, Warren LH, Hayden KM, Welsh-Bohmer KA, Breitner JCS, Morris JC. Operationalizing diagnostic criteria for Alzheimer’s disease and other age-related cognitive impairment—Part 1. Alzheimer’s Demen. 2011;7:15.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197.
Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406.
Sweet RA. Cognitive disorders: Introduction. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Philadelphia Lippincott Williams & Wilkins; 2009:1152.
Verdelho A, Madureira S, Moleiro C, Ferro JM, Santos CO, Erkinjuntti T, Pantoni L, Fazekas F, Visser M, Waldemar G, Wallin A, Hennerici M, Inzitari D. White matter changes and diabetes predict cognitive decline in the elderly: The LADIS study. Neurology. 2010;75(2):160.
Weiner MF. Cognitive disorders as psychobiological processes. In: Weiner MF, Lipton AM. The American Psychiatric Publishing Textbook of Alzheimer Disease and Other Dementias. Arlington, VA: American Psychiatric Publishing; 2009:137.
Zarit SH, Zarit JM. Disorders of aging: Delirium, dementia and other cognitive problems. In: Zarit SH, Zarit JM. Mental Disorders in Older Adults: Fundamentals of Assessment and Treatment. 2nd ed. New York: Guilford Press; 2007:40.
 21.2 Delirium
21.2 Delirium
Delirium is characterized by an acute decline in both the level of consciousness and cognition with particular impairment in attention. A life threatening, yet potentially reversible disorder of the central nervous system (CNS), delirium often involves perceptual disturbances, abnormal psychomotor activity, and sleep cycle impairment. Delirium is often underrecognized by health care workers. Part of the problem is that the syndrome has a variety of other names (Table 21.2-1).
 Table 21.2-1
Table 21.2-1
Delirium by Other Names

The hallmark symptom of delirium is an impairment of consciousness, usually occurring in association with global impairments of cognitive functions. Abnormalities of mood, perception, and behavior are common psychiatric symptoms. Tremor, asterixis, nystagmus, incoordination, and urinary incontinence are common neurological symptoms. Classically, delirium has a sudden onset (hours or days), a brief and fluctuating course, and rapid improvement when the causative factor is identified and eliminated, but each of these characteristic features can vary in individual patients. Physicians must recognize delirium to identify and treat the underlying cause and to avert the development of delirium-related complications such as accidental injury because of the patient’s clouded consciousness.
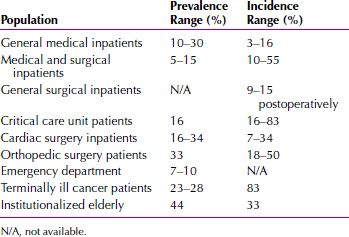
EPIDEMIOLOGY
Delirium is a common disorder, with most incidence and prevalence rates reported in elderly adults. In community studies, 1 percent of elderly persons age 55 years or older have delirium (13 percent in the age 85 years and older group in the community). Among elderly emergency department patients, 5 to 10 percent have been reported to have delirium. At the time of admission to medical wards, between 15 and 21 percent of older patients meet criteria for delirium-prevalent cases. Of patients free of delirium at time of hospital admission, 5 to 30 percent reported subsequent incidences of delirium during hospitalization. Delirium has been reported in 10 to 15 percent of general surgical patients, 30 percent of open heart surgery patients, and more than 50 percent of patients treated for hip fractures. Delirium occurs in 70 to 87 percent of those in intensive care units and in up to 83 percent of all patients at the end of life care. Sixty percent of patients in nursing homes or postacute care settings have delirium. An estimated 21 percent of patients with severe burns and 30 to 40 percent of patients with acquired immune deficiency syndrome (AIDS) have episodes of delirium while they are hospitalized. Delirium develops in 80 percent of terminally ill patients. The causes of postoperative delirium include the stress of surgery, postoperative pain, insomnia, pain medication, electrolyte imbalances, infection, fever, and blood loss. The incidence and prevalence rates for delirium across settings are shown in Table 21.2-2.
 Table 21.2-2
Table 21.2-2
Delirium Incidence and Prevalence in Multiple Settings
Risk for delirium could be conceptualized into two categories, predisposing and precipitating factors (Tables 21.2-3 and 21.2-4). Current approaches to delirium focus primarily on the precipitation factors and do little to address the predisposing factors. Managing predisposing factors for delirium becomes essential in decreasing future episodes of delirium and the morbidity and mortality associated with it.
 Table 21.2-4
Table 21.2-4
Precipitating Factors for Delirium
 Table 21.2-3
Table 21.2-3
Predisposing Factors for Delirium

Advanced age is a major risk factor for the development of delirium. Approximately 30 to 40 percent of hospitalized patients older than age 65 years have an episode of delirium, and another 10 to 15 percent of elderly persons exhibit delirium on admission to the hospital. Of nursing home residents older than age 75 years, 60 percent have repeated episodes of delirium. Male gender is also an independent risk factor for delirium.
Delirium is a poor prognostic sign. Rates of institutionalization are increased threefold for patients 65 years and older who exhibit delirium while in the hospital. The 3-month mortality rate of patients who have an episode of delirium is estimated to be 23 to 33 percent. The 1-year mortality rate for patients who have an episode of delirium may be as high as 50 percent. Elderly patients who experience delirium while hospitalized have a 21 to 75 percent mortality rate during that hospitalization. After discharge, up to 15 percent of these persons die within a 1-month period, and 25 percent die within 6 months.
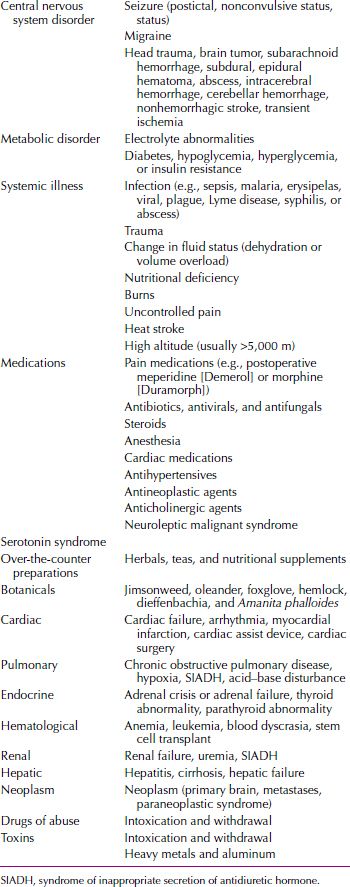
ETIOLOGY
The major causes of delirium are CNS disease (e.g., epilepsy), systemic disease (e.g., cardiac failure), and either intoxication or withdrawal from pharmacological or toxic agents (Table 21.2-5). When evaluating patients with delirium, clinicians should assume that any drug that a patient has taken may be etiologically relevant to the delirium.
 Table 21.2-5
Table 21.2-5
Common Causes of Delirium
DIAGNOSIS AND CLINICAL FEATURES
The DSM-5 diagnostic criteria for delirium are listed in Table 21.2-6. The syndrome of delirium is almost always caused by one or more systemic or cerebral derangements that affect brain function.
 Table 21.2-6
Table 21.2-6
DSM-5 Diagnostic Criteria for Delirium

A 70-year old woman, Mrs. K, was brought to the emergency department by the police. The police had responded to complaints from neighbors that Mrs. K was wandering the neighborhood and was not taking care of herself. When the police found Mrs. K in her apartment, she was dirty, foul smelling, and wearing nothing but a bra. Her apartment was also filthy with garbage and rotting food everywhere.
When interviewed, Mrs. K would not look at the interviewer and was confused and unresponsive to most of the questions asked. She knew her name and address but not the date. She was unable to describe the events that led to her admission.
The next day, the supervising psychiatrist attempted to interview Mrs. K. Her facial expression was still unresponsive, and she still did not know the month or the name of the hospital she was in. She explained that the neighbors called the police because she was “sick” and that she did indeed feel sick and weak, with pains in her shoulder. She also reported not eating for 3 days. She denied ever being in a psychiatric hospital or hearing voices but acknowledged seeing a psychiatrist at one point because she had trouble sleeping. She said the doctor had prescribed medication, but she could not remember the name.
The core features of delirium include altered consciousness, such as decreased level of consciousness; altered attention, which can include diminished ability to focus, sustain, or shift attention; impairment in other realms of cognitive function, which can manifest as disorientation (especially to time and space) and decreased memory; relatively rapid onset (usually hours to days); brief duration (usually days to weeks); and often marked, unpredictable fluctuations in severity and other clinical manifestations during the course of the day, sometimes worse at night (sundowning), which may range from periods of lucidity to severe cognitive impairment and disorganization.
Associated clinical features are often present and may be prominent. They can include disorganization of thought processes (ranging from mild tangentiality to frank incoherence), perceptual disturbances such as illusions and hallucinations, psychomotor hyperactivity and hypoactivity, disruption of the sleep–wake cycle (often manifested as fragmented sleep at night, with or without daytime drowsiness), mood alterations (from subtle irritability to obvious dysphoria, anxiety, or even euphoria), and other manifestations of altered neurological function (e.g., autonomic hyperactivity or instability, myoclonic jerking, and dysarthria). The EEG usually shows diffuse slowing of background activity, although patients with delirium caused by alcohol or sedative–hypnotic withdrawal have low-voltage fast activity.
The major neurotransmitter hypothesized to be involved in delirium is acetylcholine, and the major neuroanatomical area is the reticular formation. The reticular formation of the brainstem is the principal area regulating attention and arousal; the major pathway implicated in delirium is the dorsal tegmental pathway, which projects from the mesencephalic reticular formation to the tectum and thalamus. Several studies have reported that a variety of delirium-inducing factors result in decreased acetylcholine activity in the brain. One of the most common causes of delirium is toxicity from too many prescribed medications with anticholinergic activity. Researchers have suggested other pathophysiological mechanisms for delirium. In particular, the delirium associated with alcohol withdrawal has been associated with hyperactivity of the locus ceruleus and its noradrenergic neurons. Other neurotransmitters that have been implicated are serotonin and glutamate.
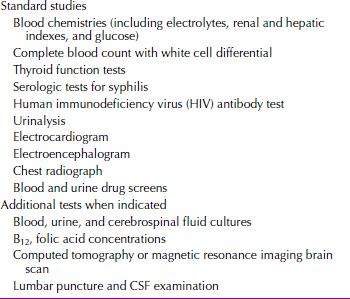
PHYSICAL AND LABORATORY EXAMINATIONS
Delirium is usually diagnosed at the bedside and is characterized by the sudden onset of symptoms. A bedside mental status examination—such as the Mini-Mental State Examination, the mental status examination, or neurological signs—can be used to document the cognitive impairment and to provide a baseline from which to measure the patient’s clinical course. The physical examination often reveals clues to the cause of the delirium (Table 21.2-7). The presence of a known physical illness or a history of head trauma or alcohol or other substance dependence increases the likelihood of the diagnosis.
 Table 21.2-7
Table 21.2-7
Physical Examination of the Delirious Patient
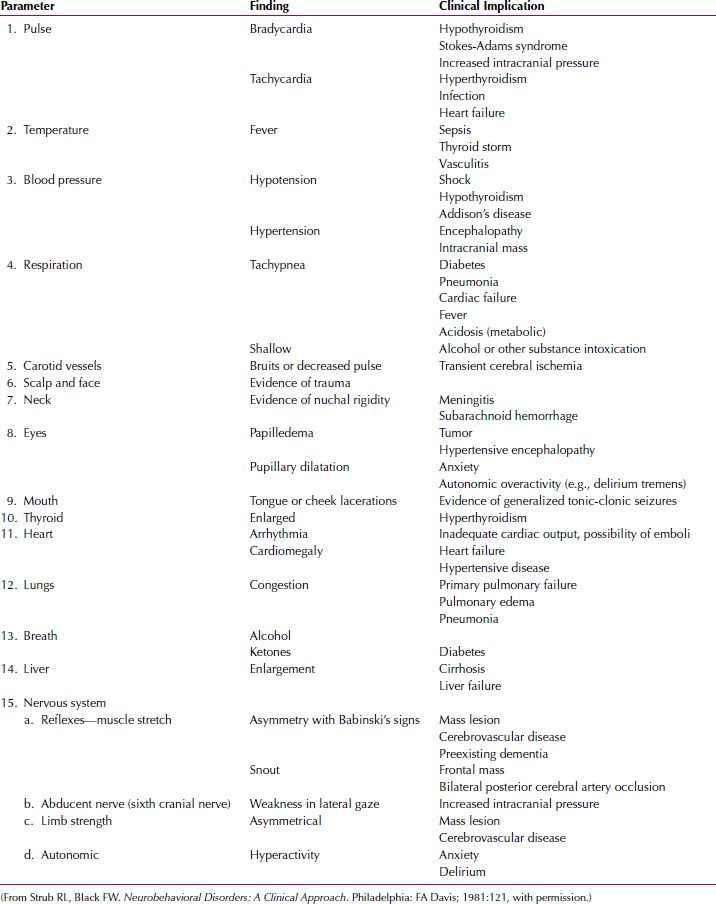
The laboratory workup of a patient with delirium should include standard tests and additional studies indicated by the clinical situation (Table 21.2-8). In delirium, the EEG characteristically shows a generalized slowing of activity and may be useful in differentiating delirium from depression or psychosis. The EEG of a delirious patient sometimes shows focal areas of hyperactivity. In rare cases, it may be difficult to differentiate delirium related to epilepsy from delirium related to other causes.
 Table 21.2-8
Table 21.2-8
Laboratory Workup of the Patient with Delirium
DIFFERENTIAL DIAGNOSIS
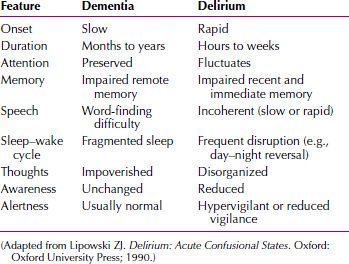
Delirium versus Dementia
A number of clinical features help distinguish delirium from dementia (Table 21.2-9). The major differential points between dementia and delirium are the time to development of the condition and the fluctuation in level of attention in delirium compared with relatively consistent attention in dementia. The time to development of symptoms is usually short in delirium, and except for vascular dementia caused by stroke, it is usually gradual and insidious in dementia. Although both conditions include cognitive impairment, the changes in dementia are more stable over time and, for example, usually do not fluctuate over the course of a day. A patient with dementia is usually alert; a patient with delirium has episodes of decreased consciousness. Occasionally, delirium occurs in a patient with dementia, a condition known as beclouded dementia. A dual diagnosis of delirium can be made when there is a definite history of preexisting dementia.
 Table 21.2-9
Table 21.2-9
Frequency of Clinical Features of Delirium Contrasted with Dementia
Delirium versus Schizophrenia or Depression
Delirium must also be differentiated from schizophrenia and depressive disorder. Some patients with psychotic disorders, usually schizophrenia or manic episodes, can have periods of extremely disorganized behavior difficult to distinguish from delirium. In general, however, the hallucinations and delusions of patients with schizophrenia are more constant and better organized than those of patients with delirium. Patients with schizophrenia usually experience no change in their level of consciousness or in their orientation. Patients with hypoactive symptoms of delirium may appear somewhat similar to severely depressed patients, but they can be distinguished on the basis of an EEG. Other psychiatric diagnoses to consider in the differential diagnosis of delirium are brief psychotic disorder, schizophreniform disorder, and dissociative disorders. Patients with factitious disorders may attempt to simulate the symptoms of delirium but usually reveal the factitious nature of their symptoms by inconsistencies on their mental status examinations, and an EEG can easily separate the two diagnoses.
COURSE AND PROGNOSIS
Although the onset of delirium is usually sudden, prodromal symptoms (e.g., restlessness and fearfulness) can occur in the days preceding the onset of florid symptoms. The symptoms of delirium usually persist as long as the causally relevant factors are present, although delirium generally lasts less than 1 week. After identification and removal of the causative factors, the symptoms of delirium usually recede over a 3- to 7-day period, although some symptoms may take up to 2 weeks to resolve completely. The older the patient and the longer the patient has been delirious, the longer the delirium takes to resolve. Recall of what transpired during a delirium, once it is over, is characteristically spotty; a patient may refer to the episode as a bad dream or a nightmare only vaguely remembered. As stated in the discussion on epidemiology, the occurrence of delirium is associated with a high mortality rate in the ensuing year, primarily because of the serious nature of the associated medical conditions that lead to delirium.
Whether delirium progresses to dementia has not been demonstrated in carefully controlled studies, although many clinicians believe that they have seen such a progression. A clinical observation that has been validated by some studies, however, is that periods of delirium are sometimes followed by depression or posttraumatic stress disorder.
TREATMENT
In treating delirium, the primary goal is to treat the underlying cause. When the underlying condition is anticholinergic toxicity, the use of physostigmine salicylate (Antilirium), 1 to 2 mg intravenously or intramuscularly, with repeated doses in 15 to 30 minutes may be indicated. The other important goal of treatment is to provide physical, sensory, and environmental support. Physical support is necessary so that delirious patients do not get into situations in which they may have accidents. Patients with delirium should be neither sensory deprived nor overly stimulated by the environment. They are usually helped by having a friend or relative in the room or by the presence of a regular sitter. Familiar pictures and decorations; the presence of a clock or a calendar; and regular orientations to person, place, and time help make patients with delirium comfortable. Delirium can sometimes occur in older patients wearing eye patches after cataract surgery (“black-patch delirium”). Such patients can be helped by placing pinholes in the patches to let in some stimuli or by occasionally removing one patch at a time during recovery.
Pharmacotherapy
The two major symptoms of delirium that may require pharmacological treatment are psychosis and insomnia. A commonly used drug for psychosis is haloperidol (Haldol), a butyrophenone antipsychotic drug. Depending on a patient’s age, weight, and physical condition, the initial dose may range from 2 to 6 mg intramuscularly, repeated in an hour if the patient remains agitated. As soon as the patient is calm, oral medication in liquid concentrate or tablet form should begin. Two daily oral doses should suffice, with two-thirds of the dose being given at bedtime. To achieve the same therapeutic effect, the oral dose should be approximately 1.5 times the parenteral dose. The effective total daily dose of haloperidol may range from 5 to 40 mg for most patients with delirium. Haloperidol has been associated with prolongation of QT interval. Clinicians should evaluate baseline and periodic electrocardiograms as well as monitor cardiac status of the patient. Droperidol (Inapsine) is a butyrophenone available as an alternative intravenous (IV) formulation, although careful monitoring of the electrocardiogram may be prudent with this treatment. The U.S. Food and Drug Administration (FDA) has issued a Black Box Warning because cases of QT prolongation and torsades de pointes have been reported in patients receiving droperidol. Because of its potential for serious proarrhythmic effects and death, it should be used only in patients who do not respond well to other treatments. Phenothiazines should be avoided in delirious patients because these drugs are associated with significant anticholinergic activity.
Use of second-generation antipsychotics, such as risperidone (Risperdal), clozapine, olanzapine (Zyprexa), quetiapine (Seroquel), ziprasidone (Geodon), and aripiprazole (Abilify), may be considered for delirium management, but clinical trial experience with these agents for delirium is limited. Ziprasidone appears to have an activating effect and may not be appropriate in delirium management. Olanzapine is available for intramuscular (IM) use and as a rapidly disintegrating oral preparation. These routes of administration may be preferable for some patients with delirium who are poorly compliant with medications or who are too sedated to safely swallow medications.
Insomnia is best treated with benzodiazepines with short or intermediate half-lives (e.g., lorazepam [Ativan] 1 to 2 mg at bedtime). Benzodiazepines with long half-lives and barbiturates should be avoided unless they are being used as part of the treatment for the underlying disorder (e.g., alcohol withdrawal). Clinicians should be aware that there is no conclusive evidence to support the use of benzodiazepines in non–alcohol-related delirium. There have been case reports of improvement in or remission of delirious states caused by intractable medical illnesses with electroconvulsive therapy (ECT); however, routine consideration of ECT for delirium is not advised. If delirium is caused by severe pain or dyspnea, a physician should not hesitate to prescribe opioids for both their analgesic and sedative effects (Table 21.2-10).
 Table 21.2-10
Table 21.2-10
Pharmacological Treatment
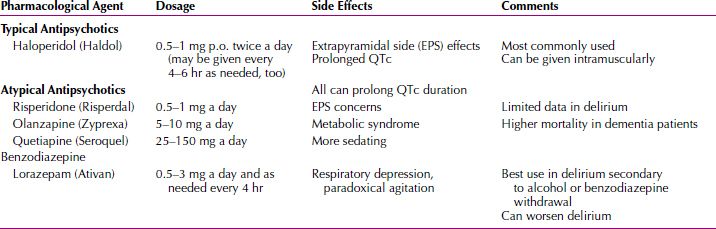
Current trials are ongoing to see if dexmedetomidine (Precedex) is a more effective medication than haloperidol in the treatment of agitation and delirium in patients receiving mechanical ventilation in an intensive care unit.
Treatment in Special Populations
Parkinson’s Disease. In Parkinson’s disease, the antiparkinsonian agents are frequently implicated in causing delirium. If a coexistent dementia is present, delirium is twice as likely to develop in patients with Parkinson’s disease with dementia receiving antiparkinsonian agents than in those without dementia. Decreasing the dosage of the antiparkinsonian agent has to be weighed against a worsening of motor symptoms. If the antiparkinsonian agents cannot be further reduced, or if the delirium persists after attenuation of the antiparkinsonian agents, clozapine is recommended. If a patient is not able to tolerate clozapine or the required blood monitoring, alternative antipsychotic agents should be considered. Quetiapine has not been as rigorously studied as clozapine and may have parkinsonian side effects, but it is used in clinical practice to treat psychosis in Parkinson’s disease.
Terminally Ill Patients. When delirium occurs in the context of a terminal illness, issues about advanced directives and the existence of a health care proxy become more significant. This scenario emphasizes the importance of early development of advance directives for health care decision making while a person has the capacity to communicate the wishes regarding the extent of aggressive diagnostic tests at life’s end. The focus may change from an aggressive search for the etiology of the delirium to one of palliation, comfort, and assistance with dying.
REFERENCES
Caraceni A, Grassi L. Delirium: Acute Confusional States in Palliative Medicine. 2nd ed. New York: Oxford University Press; 2111.
Franco JG, Trzepacz PT, Meagher DJ, Kean J, Lee Y, Kim J-L, Kishi Y, Furlanetto LM, Negreiros D, Huang M-C, Chen C-H, Leonard M, de Pablo J. Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics. 2113;54:227.
Hosie A, Davidson PM, Agar M, Sanderson CR, Philips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: A systematic review. Palliative Med. 2113;27:486.
Juliebö V, Björo K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2109;57:1354.
Kiely DK, Marcantonio ER, Inouye SK, Shaffer ML, Bergmann MA, Yang FM, Fearing MA, Jones RN. Persistent delirium predicts greater mortality. J Am Geriatr Soc. 2109;57:55.
Maldonado JR, Wysong A, van der Starre PJA, Block T, Miller C, Reitz BA. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2109;50:216.
Morandi A, McCurley J, Vasilevskis EE. Tools to detect delirium superimposed on dementia: A systematic review: Erratum. J Am Ger Soc. 2113;61:174.
O’Mahony R, Murthy L, Akunne A, Young J. Synopsis of the National Institute for Health and Clinical Excellence guideline for prevention of delirium. Ann Intern Med. 2111;154(11):746.
Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KLB, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care. 2109;180:1092.
Popeo DM. Delirium in older adults. MT Sinai J Med. 2111;78(4):571.
Singh Joy SD. Delirium directly related to cognitive impairment. Am J Nurs. 2111;111:65.
Solai LKK. Delirium. In: Sadock BJ, Sadock VA, Ruiz P, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2109:1153.
Thomas E, Smith JE, Forrester DA, Heider G, Jadotte YT, Holly C. The effectiveness of non-pharmacological multi-component interventions for the prevention of delirium in non-intensive care unit older adult hospitalized patients: a systematic review. The JBI Database of Systematic Reviews and Implementation Reports. 2014;12(4):180–232.
Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia. JAMA. 2110;304(4):443.
Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA, Jones RN. Phenomenological subtypes of delirium in older persons: Patterns, prevalence, and prognosis. Psychosomatics. 2109;50:248.
 21.3 Dementia (Major Neurocognitive Disorder)
21.3 Dementia (Major Neurocognitive Disorder)
Dementia refers to a disease process marked by progressive cognitive impairment in clear consciousness. Dementia does not refer to low intellectual functioning or mental retardation because these are developmental and static conditions, and the cognitive deficits in dementia represent a decline from a previous level of functioning. Dementia involves multiple cognitive domains and cognitive deficits cause significant impairment in social and occupational functioning. There are four types of dementias based on etiology: Alzheimer’s disease, dementia of Lewy bodies, vascular dementia, frontotemporal dementia, traumatic brain injury (TBI), HIV, prion disease, Parkinson’s disease, and Huntington’s disease. Dementia can also be caused by other medical and neurological conditions or can be caused by various substances. (See Section 21.4: Amnestic Disorders.)
The critical clinical points of dementia are the identification of the syndrome and the clinical workup of its cause. The disorder can be progressive or static; permanent or reversible. An underlying cause is always assumed, although, in rare cases, it is impossible to determine a specific cause. The potential reversibility of dementia is related to the underlying pathological condition and to the availability and application of effective treatment. Approximately 15 percent of people with dementia have reversible illnesses if treatment is initiated before irreversible damage takes place.
EPIDEMIOLOGY
With the aging population, the prevalence of dementia is rising. The prevalence of moderate to severe dementia in different population groups is approximately 5 percent in the general population older than 65 years of age, 20 to 40 percent in the general population older than 85 years of age, 15 to 20 percent in outpatient general medical practices, and 50 percent in chronic care facilities.
Of all patients with dementia, 50 to 60 percent have the most common type of dementia, dementia of the Alzheimer’s type (Alzheimer’s disease). Dementia of the Alzheimer’s type increases in prevalence with increasing age. For persons age 65 years, men have a prevalence rate of 0.6 percent and women of 0.8 percent. At age 90, rates are 21 percent. For all of these figures, 40 to 60 percent of cases are moderate to severe. The rates of prevalence (men to women) are 11 and 14 percent at age 85, 21 and 25 percent at age 90 years and 36 and 41 percent at age 95 years. Patients with dementia of the Alzheimer’s type occupy more than 50 percent of nursing home beds. More than 2 million persons with dementia are cared for in these homes. By 2050, current predictions suggest that there will be 14 million Americans with Alzheimer’s disease and therefore more than 18 million people with dementia.
The second most common type of dementia is vascular dementia, which is causally related to cerebrovascular diseases. Hypertension predisposes a person to the disease. Vascular dementias account for 15 to 30 percent of all dementia cases. Vascular dementia is most common in persons between the ages of 60 and 70 and is more common in men than in women. Approximately 10 to 15 percent of patients have coexisting vascular dementia and dementia of the Alzheimer’s type.
Other common causes of dementia, each representing 1 to 5 percent of all cases, include head trauma; alcohol-related dementias; and various movement disorder-related dementias, such as Huntington’s disease and Parkinson’s disease. Because dementia is a fairly general syndrome, it has many causes, and clinicians must embark on a careful clinical workup of a patient with dementia to establish its cause.
ETIOLOGY
The most common causes of dementia in individuals older than 65 years of age are (1) Alzheimer’s disease, (2) vascular dementia, and (3) mixed vascular and Alzheimer’s dementia. Other illnesses that account for approximately 10 percent include Lewy body dementia; Pick’s disease; frontotemporal dementias; normal-pressure hydrocephalus (NPH); alcoholic dementia; infectious dementia, such as HIV or syphilis; and Parkinson’s disease. Many types of dementias evaluated in clinical settings can be attributable to reversible causes, such as metabolic abnormalities (e.g., hypothyroidism), nutritional deficiencies (e.g., vitamin B12 or folate deficiencies), or dementia syndrome caused by depression. See Table 21.3-1 for a review of possible etiologies of dementia.
 Table 21.3-1
Table 21.3-1
Possible Etiologies of Dementia
Dementia of the Alzheimer’s Type
In 1907, Alois Alzheimer (Fig. 21.3-1) first described the condition that later assumed his name. He described a 51-year-old woman with a 4½-year course of progressive dementia. The final diagnosis of Alzheimer’s disease requires a neuropathological examination of the brain; nevertheless, dementia of the Alzheimer’s type is commonly diagnosed in the clinical setting after other causes of dementia have been excluded from diagnostic consideration.

FIGURE 21.3-1
Alois Alzheimer (1864–1915), a German psychiatrist, described a type of senile dementia that bears his name
Genetic Factors. Although the cause of dementia of the Alzheimer’s type remains unknown, progress has been made in understanding the molecular basis of the amyloid deposits that are a hallmark of the disorder’s neuropathology. Some studies have indicated that as many as 40 percent of patients have a family history of dementia of the Alzheimer’s type; thus, genetic factors are presumed to play a part in the development of the disorder, at least in some cases. Additional support for a genetic influence is the concordance rate for monozygotic twins, which is higher than the rate for dizygotic twins (43 percent vs. 8 percent, respectively). In several well-documented cases, the disorder has been transmitted in families through an autosomal dominant gene, although such transmission is rare. Alzheimer’s type dementia has shown linkage to chromosomes 1, 14, and 21.
AMYLOID PRECURSOR PROTEIN. The gene for amyloid precursor protein is on the long arm of chromosome 21. The process of differential splicing yields four forms of amyloid precursor protein. The β/A4 protein, the major constituent of senile plaques, is a 42-amino acid peptide that is a breakdown product of amyloid precursor protein. In Down syndrome (trisomy 21) are found three copies of the amyloid precursor protein gene, and in a disease in which a mutation is found at codon 717 in the amyloid precursor protein gene, a pathological process results in the excessive deposition of β/A4 protein. Whether the processing of abnormal amyloid precursor protein is of primary causative significance in Alzheimer’s disease is unknown, but many research groups are studying both the normal metabolic processing of amyloid precursor protein and its processing in patients with dementia of the Alzheimer’s type in an attempt to answer this question.
MULTIPLE E4 GENES. One study implicated gene E4 in the origin of Alzheimer’s disease. People with one copy of the gene have Alzheimer’s disease three times more frequently than do those with no E4 gene, and people with two E4 genes have the disease eight times more frequently than do those with no E4 gene. Diagnostic testing for this gene is not currently recommended because it is found in persons without dementia and not found in all cases of dementia.
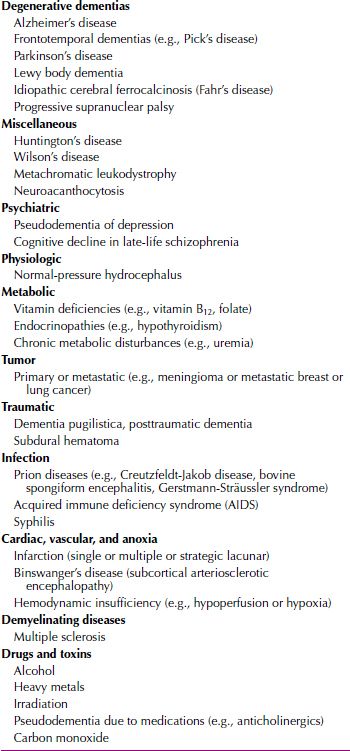
Neuropathology. The classic gross neuroanatomical observation of a brain from a patient with Alzheimer’s disease is diffuse atrophy with flattened cortical sulci and enlarged cerebral ventricles. The classic and pathognomonic microscopic findings are senile plaques, neurofibrillary tangles, neuronal loss (particularly in the cortex and the hippocampus), synaptic loss (perhaps as much as 50 percent in the cortex), and granulovascular degeneration of the neurons. Neurofibrillary tangles (Fig. 21.3-2) are composed of cytoskeletal elements, primarily phosphorylated tau protein, although other cytoskeletal proteins are also present. Neurofibrillary tangles are not unique to Alzheimer’s disease; they also occur in Down syndrome, dementia pugilistica (punch-drunk syndrome), Parkinson-dementia complex of Guam, Hallervorden-Spatz disease, and the brains of normal people as they age. Neurofibrillary tangles are commonly found in the cortex, the hippocampus, the substantia nigra, and the locus ceruleus.
FIGURE 21.3-2
Photomicrographs of Alzheimer’s disease neuropathology. (A) Deposition of insoluble fibrillar Aβ into plaques begins in the neocortex, labeled here using an antibody against Aβ and appearing as reddish-brown deposits (arrows). (B) Bielchowsky stain of neocortex from an individual who died in advanced stages of Alzheimer’s disease (Braak stage VI). The Aβ plaques appear as dark brown in this preparation (arrows) and can be seen to be associated with dystrophic neuronal processes (arrowheads) in which insoluble microtubule-associate protein τ (MAPT) aggregates appear as black deposits. This neurofibrillary pathology also appears extensively throughout the neuropil, and several neurofibrillary tangles can be seen (open arrowheads). (C) Bielchowsky stain of neocortex from an individual who died in a less advanced disease stage (Braak stage IV). Although some neurofibrillary tangles are still evident (open arrowheads), the degree of neurofibrillary pathology in the neuropil is substantially diminished. (D) Isolated neurofibrillary tangles (open arrowheads) in entorhinal cortex that can be seen in normal aging (Bielchowsky stain). Notice the lack of Aβ plaques and limited neuropil involvement. (All images obtained at 200× magnification and provided courtesy of Dr. Ronald L. Hamilton, Department of Pathology, Division of Neuropathology, University of Pittsburgh School of Medicine.)
Senile plaques, also referred to as amyloid plaques, more strongly indicate Alzheimer’s disease, although they are also seen in Down syndrome and, to some extent, in normal aging. Senile plaques are composed of a particular protein, β/A4, and astrocytes, dystrophic neuronal processes, and microglia. The number and the density of senile plaques present in postmortem brains have been correlated with the severity of the disease that affected the persons.
Neurotransmitters. The neurotransmitters that are most often implicated in the pathophysiological condition of Alzheimer’s disease are acetylcholine and norepinephrine, both of which are hypothesized to be hypoactive in Alzheimer’s disease. Several studies have reported data consistent with the hypothesis that specific degeneration of cholinergic neurons is present in the nucleus basalis of Meynert in persons with Alzheimer’s disease. Other data supporting a cholinergic deficit in Alzheimer’s disease demonstrate decreased acetylcholine and choline acetyltransferase concentrations in the brain. Choline acetyltransferase is the key enzyme for the synthesis of acetylcholine, and a reduction in choline acetyltransferase concentration suggests a decrease in the number of cholinergic neurons present. Additional support for the cholinergic deficit hypothesis comes from the observation that cholinergic antagonists, such as scopolamine and atropine, impair cognitive abilities, whereas cholinergic agonists, such as physostigmine and arecoline, enhance cognitive abilities. Decreased norepinephrine activity in Alzheimer’s disease is suggested by the decrease in norepinephrine-containing neurons in the locus ceruleus found in some pathological examinations of brains from persons with Alzheimer’s disease. Two other neurotransmitters implicated in the pathophysiological condition of Alzheimer’s disease are the neuroactive peptides somatostatin and corticotropin; decreased concentrations of both have been reported in persons with Alzheimer’s disease.
Other Causes. Another theory to explain the development of Alzheimer’s disease is that an abnormality in the regulation of membrane phospholipid metabolism results in membranes that are less fluid—that is, more rigid—than normal. Several investigators are using molecular resonance spectroscopic imaging to assess this hypothesis directly in patients with dementia of the Alzheimer’s type. Aluminum toxicity has also been hypothesized to be a causative factor because high levels of aluminum have been found in the brains of some patients with Alzheimer’s disease, but this is no longer considered a significant etiological factor. Excessive stimulation by the transmitter glutamate that may damage neurons is another theory of causation.
Familial Multiple System Taupathy with Presenile Dementia. A recently discovered type of dementia, familial multiple system taupathy, shares some brain abnormalities found in people with Alzheimer’s disease. The gene that causes the disorder is thought to be carried on chromosome 17. The symptoms of the disorder include short-term memory problems and difficulty maintaining balance and walking. The onset of disease occurs in the 40s and 50s, and persons with the disease live an average of 11 years after the onset of symptoms.
As in patients with Alzheimer’s disease, tau protein builds up in neurons and glial cells of persons with familial multiple system taupathy. Eventually, the protein buildup kills brain cells. The disorder is not associated with the senile plaques seen with Alzheimer’s disease.
Mr. J, a 70-year-old retired businessman, was brought to psychiatric services on referral by the family physician. His wife claimed that Mr. J had become so forgetful that she was afraid to leave him alone, even at home. Mr. J retired at age 62 years after experiencing a decline in work performance during the previous 5 years. He also slowly gave up hobbies he once enjoyed (photography, reading, golf) and became increasingly quiet. However, his growing forgetfulness went basically unnoticed at home. Then one day while walking in an area he knew well, he could not find his way home. From then on his memory failure began to increase. He would forget appointments, misplace things, and lose his way around the neighborhood he resided in for 40 years. He failed to recognize people, even those he knew for many years. His wife had to start bathing and dressing him because he forgot how to do so himself.
On examination, Mr. J was disoriented in time and place. He was only able to recall his name and place of birth. Mr. J seemed lost during the interview, only responding to questions with an occasional shrug of his shoulders. When asked to name objects or to recall words or numbers, Mr. J appeared tense and distressed. Mr. J had difficulty following instructions and was unable to dress or undress himself. His general medical condition was good. Laboratory examinations showed abnormalities on Mr. J’s EEG and CT scans.
Vascular Dementia
The primary cause of vascular dementia, formerly referred to as multi-infarct dementia, is presumed to be multiple areas of cerebral vascular disease, resulting in a symptom pattern of dementia. Vascular dementia most commonly is seen in men, especially those with preexisting hypertension or other cardiovascular risk factors. The disorder affects primarily small- and medium-sized cerebral vessels, which undergo infarction and produce multiple parenchymal lesions spread over wide areas of the brain (Fig. 21.3-3). The causes of the infarctions can include occlusion of the vessels by arteriosclerotic plaques or thromobemboli from distant origins (e.g., heart valves). An examination of a patient may reveal carotid bruits, funduscopic abnormalities, or enlarged cardiac chambers (Fig. 21.3-4).
FIGURE 21.3-4
Patients with chronic dementia usually requires custodial care in their declining years. Regressive behavior, such as finger sucking, is typical in this state. (Courtesy of Bill Stanton for Magnum Photos, Inc.)
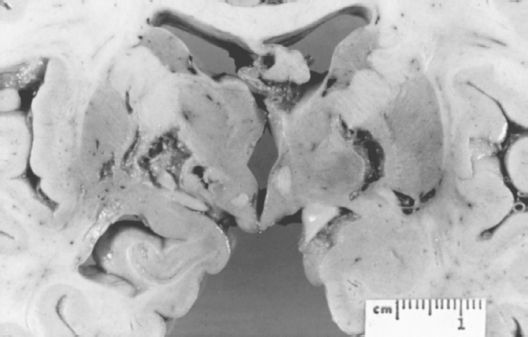
FIGURE 21.3-3
Gross appearance of the cerebral cortex on coronal section from a patient with vascular dementia. The multiple bilateral lacunar infarcts involve the thalamus, the internal capsule, and the globus pallidus. (Courtesy of Daniel P. Perl, M.D.)
Binswanger’s Disease. Binswanger’s disease (Fig. 21.3-5), also known as subcortical arteriosclerotic encephalopathy, is characterized by the presence of many small infarctions of the white matter that spare the cortical regions (Fig. 21.3-6). Although Binswanger’s disease was previously considered a rare condition, the advent of sophisticated and powerful imaging techniques, such as MRI, has revealed that the condition is more common than previously thought.
FIGURE 21.3-5
Otto Binswanger (1852–1929), a Swiss psychiatrist who described a condition he call “encephalitis subcorticalis chronica progressive,” now known as Binswanger’s disease.
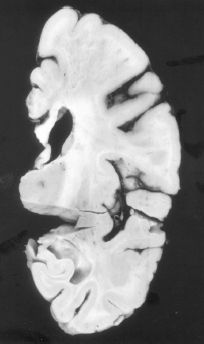
FIGURE 21.3-6
Binswanger’s disease. Cross-section demonstrating extensive subcortical white matter infarction, with sparing of the overlying gray matter. (Courtesy of Dushyant Purohit, M.D., Neuropathology Division, Mount Sinai School of Medicine, New York, NY.)
Frontotemporal Dementia (Pick’s Disease)
In contrast to the parietal-temporal distribution of pathological findings in Alzheimer’s disease, Pick’s disease (Fig. 21.3-7) is characterized by a preponderance of atrophy in the frontotemporal regions. These regions also have neuronal loss; gliosis; and neuronal Pick’s bodies, which are masses of cytoskeletal elements. Pick’s bodies are seen in some postmortem specimens but are not necessary for the diagnosis. The cause of Pick’s disease is unknown, but the disease constitutes approximately 5 percent of all irreversible dementias. It is most common in men, especially those who have a first-degree relative with the condition. Pick’s disease is difficult to distinguish from dementia of the Alzheimer’s type, although the early stages of Pick’s disease are more often characterized by personality and behavioral changes, with relative preservation of other cognitive functions, and it typically begins before 75 years of age. Familial cases may have an earlier onset, and some studies have shown that approximately half of the cases of Pick’s disease are familial (Fig. 21.3-8). Features of Klüver-Bucy syndrome (e.g., hypersexuality, placidity, and hyperorality) are much more common in Pick’s disease than in Alzheimer’s disease.

FIGURE 21.3-7
Arnold Pick (1851–1924), a Czech neurologist and psychiatrist who described frontotemporal dementia and the Pick bodies that are characteristic of the disorder.
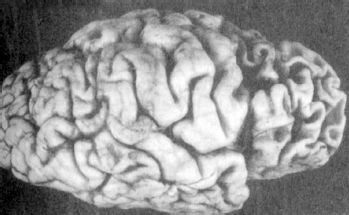
FIGURE 21.3-8
Pick’s disease gross pathology. This demonstrates the marked frontal and temporal atrophy seen in frontotemporal dementias, such as Pick’s disease. (Courtesy of Dushyant Purohit, M.D., Neuropathology Division, Mount Sinai School of Medicine, New York, NY.)
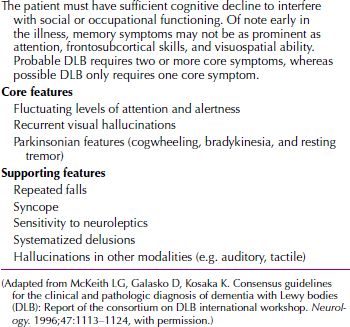
Lewy Body Disease
Lewy body disease is a dementia clinically similar to Alzheimer’s disease and often characterized by hallucinations, parkinsonian features, and extrapyramidal signs (Table 21.3-2). Lewy inclusion bodies are found in the cerebral cortex (Fig. 21.3-9). The exact incidence is unknown. These patients often have Capgras syndrome (reduplicative paramnesia) as party of the clinical picture.
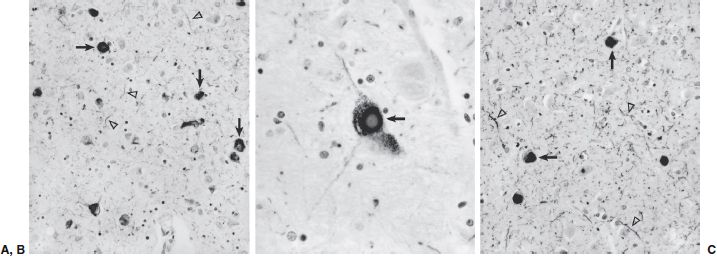
FIGURE 21.3-9
Photomicrographs of Lewy body pathology. (A) Abnormal accumulation of α-synuclein aggregates demonstrated by immunocytochemistry in the amygdala of a subject with dementia. Lewy bodies appear as dense intracellular inclusions (arrows), but staining of neuronal processes can be seen throughout the neuropil (arrowheads). In individuals in whom Lewy body pathology occurs concurrently with Alzheimer’s disease, the amygdala is often the only region affected. (B) Classic appearance of a Lewy body (arrow) in a large pigmented neuron of the substantia nigra. (C) Lewy body pathology in the neocortex. Both Lewy bodies (arrows) and substantial labeling of neuronal processes in the neuropil (arrowheads) are evident. (Magnification for [A] and [B] 200×, for [C] 400×. All images provided courtesy of Dr. Ronald L. Hamilton, Department of Pathology, Division of Neuropathology, University of Pittsburgh School of Medicine.)
 Table 21.3-2
Table 21.3-2
Clinical Criteria for Dementia with Lewy Bodies (DLB)
Huntington’s Disease
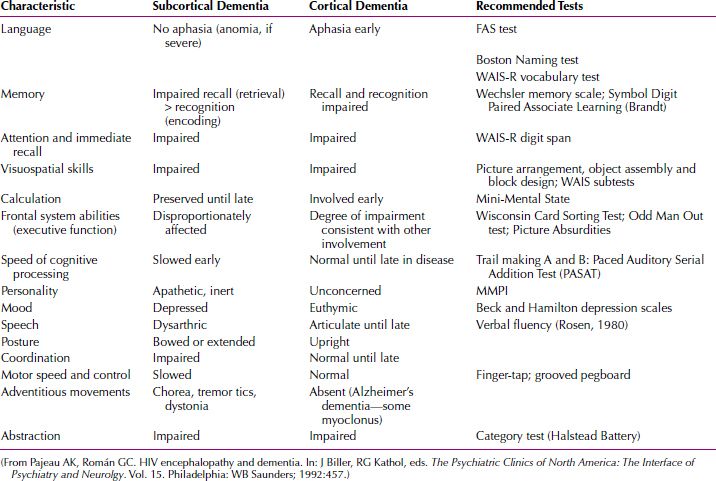
Huntington’s disease (Fig. 21.3-10) is classically associated with the development of dementia. The dementia seen in this disease is the subcortical type of dementia, characterized by more motor abnormalities and fewer language abnormalities than in the cortical type of dementia (Table 21.3-3). The dementia of Huntington’s disease exhibits psychomotor slowing and difficulty with complex tasks, but memory, language, and insight remain relatively intact in the early and middle stages of the illness. As the disease progresses, however, the dementia becomes complete; the features distinguishing it from dementia of the Alzheimer’s type are the high incidence of depression and psychosis in addition to the classic choreoathetoid movement disorder.

FIGURE 21.3-10
George Huntington (1850–1916), an American physician who first described the disease that bears his name, Huntington’s disease.
 Table 21.3-3
Table 21.3-3
Distinguishing Features of Subcortical and Cortical Dementias
Parkinson’s Disease
As with Huntington’s disease, parkinsonism is a disease of the basal ganglia, commonly associated with dementia and depression. An estimated 20 to 30 percent of patients with Parkinson’s disease have dementia, and an additional 30 to 40 percent have measurable impairment in cognitive abilities. The slow movements of persons with Parkinson’s disease are paralleled in the slow thinking of some affected patients, a feature that clinicians may refer to as bradyphrenia.
Mr. M, 77 years of age, came for a neurological examination because he noticed his memory was slipping and he was having difficulty concentrating, which interfered with his work. He complained of slowness and losing his train of thought. His wife stated that he was becoming withdrawn and was more reluctant to participate in activities he usually enjoyed. He denied symptoms of depression other than feeling mildly depressed about his disabilities. Two years prior, Mr. M developed an intermittent resting tremor in his right hand and a shuffling gait. Although a psychiatrist considered a diagnosis of Parkinson’s disease, it was not confirmed by a neurologist and therefore was never treated.
During an initial neurological examination, Mr. M’s spontaneous speech was hesitant and unclear (dysarthric). Cranial nerve examination was normal. Motor tone was increased slightly in the neck and all limbs. He performed alternating movements in his hands slowly. He had a slight intermittent tremor of his right arm at rest. Reflexes were symmetrical. A neuropsychological examination was performed three weeks later. It was found that Mr. M showed impairment of memory, naming, and constructional abilities.
HIV-Related Dementia
Encephalopathy in HIV infection is associated with dementia and is termed acquired immune deficiency syndrome (AIDS) dementia complex, or HIV dementia. Patients infected with HIV experience dementia at an annual rate of approximately 14 percent. An estimated 75 percent of patients with AIDS have involvement of the CNS at the time of autopsy. The development of dementia in people infected with HIV is often paralleled by the appearance of parenchymal abnormalities in MRI scans. Other infectious dementias are caused by Cryptococcus or Treponema pallidum.
The diagnosis of AIDS dementia complex is made by confirmation of HIV infection and exclusion of alternative pathology to explain cognitive impairment. The American Academy of Neurology AIDS Task Force developed research criteria for the clinical diagnosis of CNS disorders in adults and adolescents (Table 21.3-4). The AIDS Task Force criteria for AIDS dementia complex require laboratory evidence for systemic HIV, at least two cognitive deficits, and the presence of motor abnormalities or personality changes. Personality changes may be manifested by apathy, emotional lability, or behavioral disinhibition. The AIDS Task Force criteria also require the absence of clouding of consciousness or evidence of another etiology that could produce the cognitive impairment. Cognitive, motor, and behavioral changes are assessed using physical, neurological, and psychiatric examinations, in addition to neuropsychological testing.
 Table 21.3-4
Table 21.3-4
Criteria for Clinical Diagnosis of HIV Type 1-Associated Dementia Complex

Head Trauma-Related Dementia
Dementia can be a sequela of head trauma. The so-called punch-drunk syndrome (dementia pugilistica) occurs in boxers after repeated head trauma over many years. It is characterized by emotional lability, dysarthria, and impulsivity. It has also been observed in professional football players who developed dementia after repeated concussions over many years.
Mrs. S, 75 years of age, was brought to the emergency department after being found wandering her neighborhood in a confused and disoriented state. She was in good health until a few months prior when her husband was hospitalized for 10 days for minor surgery. About a month after her husband returned home, he and their two adult children, who do not reside with them, reported a noticeable change in Mrs. S’s mental status. Mrs. S became hyperactive and appeared to have excessive energy, was agitated and irritable, and had difficulty sleeping at night.
At examination, Mrs. S was disoriented to time and place, agitated, and confused. Her husband revealed upon interview that Mrs. S has for many years suffered from dizziness and lightheadedness upon standing and occasionally suffered from falls, none of which caused any major damage. Not long before her confused symptoms began, Mrs. S had apparently suffered a fall one night, and her husband found her the next morning lying next to the bed in a confused state. Because of her history of falls, neither Mr. S nor Mrs. S thought much of the incident. A CT scan revealed the presence of a subdural hematoma, which was then evacuated. Afterward, Mrs. S’s confusion and disorientation cleared and she returned to her normal state of functioning.
DIAGNOSIS AND CLINICAL FEATURES
The DSM-5 diagnostic criteria are listed in Tables 21.3-5 and 21.3-6. DSM-5 makes a distinction between major and minor cognitive disorder based upon levels of functioning, but the underlying etiology is similar.
 Table 21.3-5
Table 21.3-5
DSM-5 Diagnostic Criteria for Major Neurocognitive Disorder (Dementia)
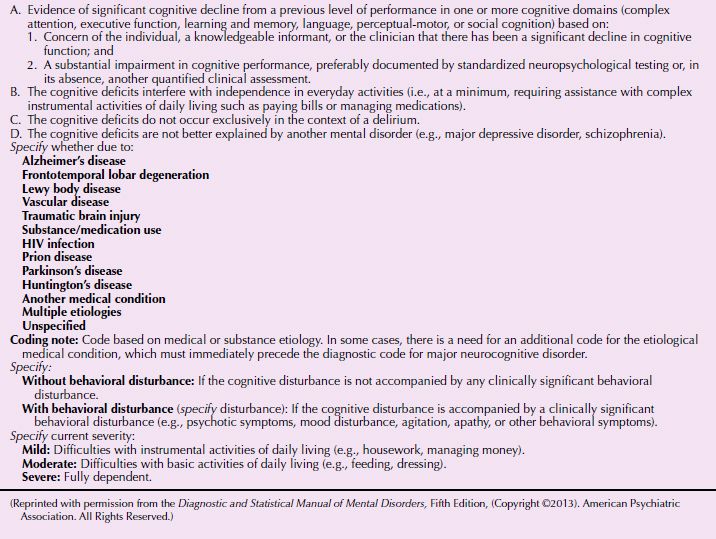
 Table 21.3-6
Table 21.3-6
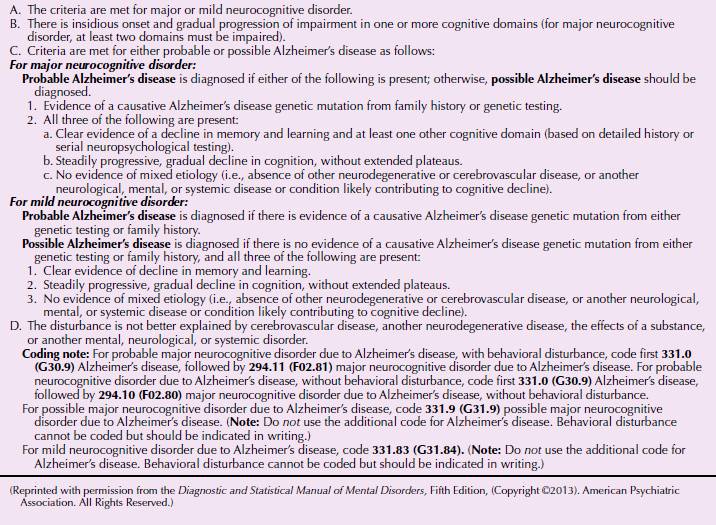
DSM-5 Diagnostic Criteria for Major or Mild Neurocognitive Disorder Due to Alzheimer’s Disease
The diagnosis of dementia is based on the clinical examination, including a mental status examination, and on information from the patient’s family, friends, and employers. Complaints of a personality change in a patient older than age 40 years suggest that a diagnosis of dementia should be carefully considered.
Clinicians should note patients’ complaints about intellectual impairment and forgetfulness as well as evidence of patients’ evasion, denial, or rationalization aimed at concealing cognitive deficits. Excessive orderliness, social withdrawal, or a tendency to relate events in minute detail can be characteristic, and sudden outbursts of anger or sarcasm can occur. Patients’ appearance and behavior should be observed. Lability of emotions; sloppy grooming; uninhibited remarks; silly jokes; or a dull, apathetic, or vacuous facial expression and manner suggest the presence of dementia, especially when coupled with memory impairment.
Memory impairment is typically an early and prominent feature in dementia, especially in dementias involving the cortex, such as dementia of the Alzheimer’s type. Early in the course of dementia, memory impairment is mild and usually most marked for recent events; people forget telephone numbers, conversations, and events of the day. As the course of dementia progresses, memory impairment becomes severe, and only the earliest learned information (e.g., a person’s place of birth) is retained.
Inasmuch as memory is important for orientation to person, place, and time, orientation can be progressively affected during the course of a dementing illness. For example, patients with dementia may forget how to get back to their rooms after going to the bathroom. No matter how severe the disorientation seems, however, patients show no impairment in their level of consciousness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








