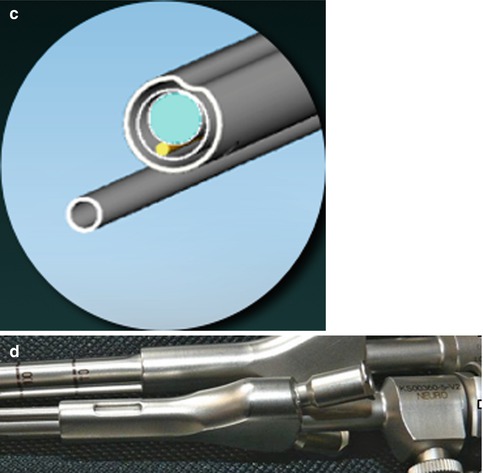
Fig. 13.1
(a–c) Depict the sheaths covering the scope harboring suction and dissecting tools constructed for 0° and 30° scopes. The sulcus present in the shaft guides additional instruments to the operating site. Continuous aspiration close to the lens provides clean vision. (d) Shows the handle cleft meant to control the suction intensity
The suction tube may act as a second dissecting tool when the optimized suction intensity control is left opened.
13.5 Surgical Technique
After defining the ideal trajectory, a burr hole is enlarged to 12 mm. For superficial lesion additional removal of 1–2 mm of the inner cortical bone of the skull may ease lateral movements. An arciform dural incision facilitates closure.
Two techniques may be used to create a tunnel to reach the lesion:
1.
A cannula with a blunt tip obturator is introduced to the limit between the normal brain and lesion. After removing the obturator, visual identification of abnormal tissue is possible, and sampling for intraoperative histologic confirmation may be obtained. The cannulas vary from 70 to 110 mm in length and have 9-mm external diameter. Dissection can be performed through the cannula, when several instruments may be used simultaneously under endoscopic view. A wider range of movements are obtained by removing the cannula and working through the parenchymal tunnel left, which will remain open with a diameter of 7–8 mm. The maintenance of the opening of the tunnel may be benefited by anesthesia techniques and a vertical trajectory.
2.
A direct access by blunt dissection can also lead to the lesion. As mentioned above, working without a cannula gives additional liberty of movements. In this situation typical bimanual neurosurgical microdissection is accomplished under bright illumination and high-definition imaging provided by the scope.
In typical cases, bleeding is very limited, as strong illumination, image amplification, and wide-angle lens allow the vessels to be coagulated before they bleed. When working through a bare tunnel, conventional bipolar coagulation ensures safe hemostasis.
This technique reduces incision and damage to the skin, bone, and dura mater, cutting down surgical time and costs, due to opening and closing of surgical wounds.
Once the scope brings light and vision to the tip of the instruments, brain retraction necessary to visualize from outside to the operating field is obviated.
The experience, taken from 81 selected cases, including primary and secondary brain tumors, cavernous angiomas, intraparenchymal hematomas, cerebellar infarctions, sellar tumors, and brain abscess, proves the technique as an accurate and safe procedure. Except for a transient third nerve paresis in a patient with mesial temporal metastasis, no additional neurological deficit was produced in this series of patients.
The patients treated with this method had a shorter ICU and hospital stay when compared to similar cases treated by microsurgical technique by the same group of surgeons.
13.6 Illustrated Cases
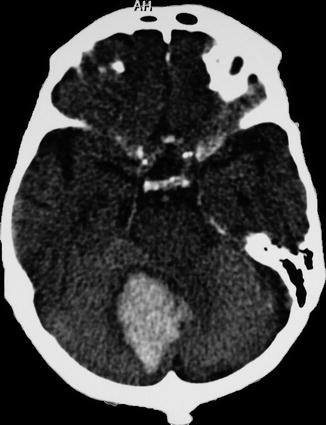
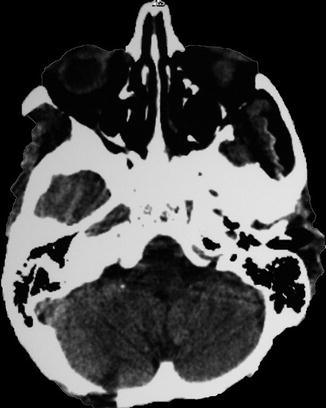
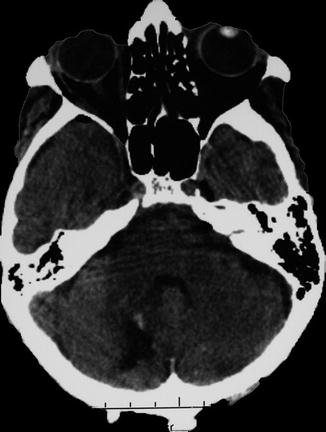
13.6.1 Intraparenchymatous Hematoma
13.6.1.1 Case 1 (Fig.s. 13.2, 13.3, 13.4, and 13.5)
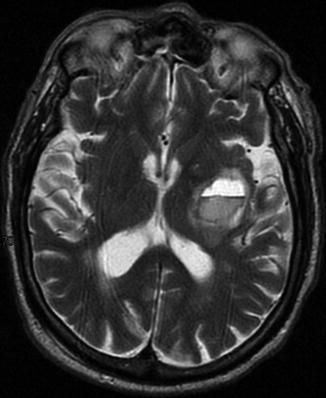
Fig. 13.2
Left putaminal hematoma compressing the posterior limb of the internal capsule
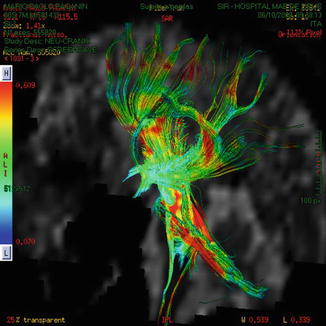
Fig. 13.3
The corticospinal fibers surround the clot. A posterior frontal approach was selected in order to avoid interrupting motor tracts
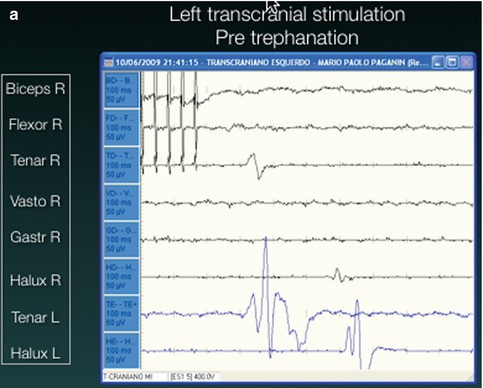
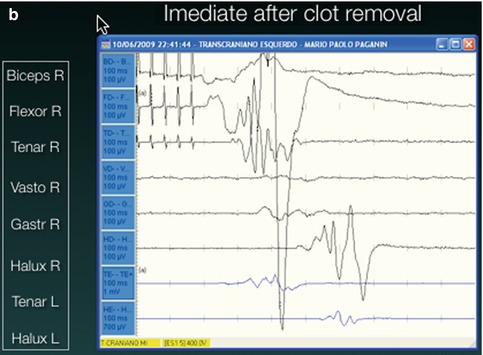
Fig. 13.4
Intraoperative motor monitoring. In (a) there is no motor response to transcranial stimulation. (b) Immediate recordings after clot aspiration demonstrated recovery of the motor responses
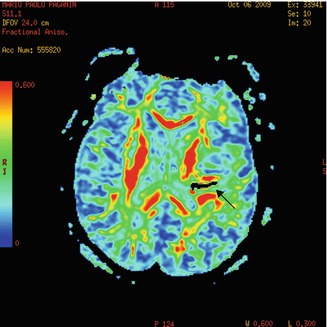
Fig. 13.5
Immediate postop DTI depicts decompression of motor tract. The black arrow shows air into the hematoma cavity
13.6.2 Brain Glioma (Fig.s. 13.9, 13.10, 13.11, and 13.12)
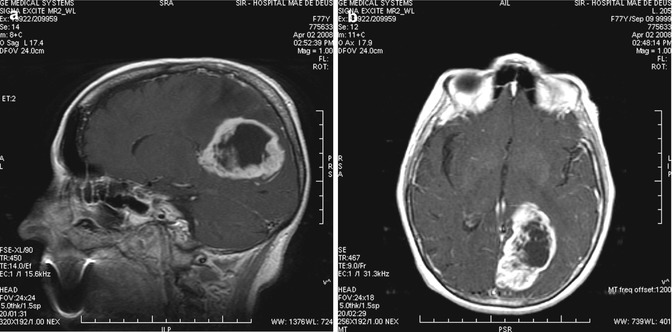
Fig. 13.9
(a, b) Preoperative images of left occipital GBM
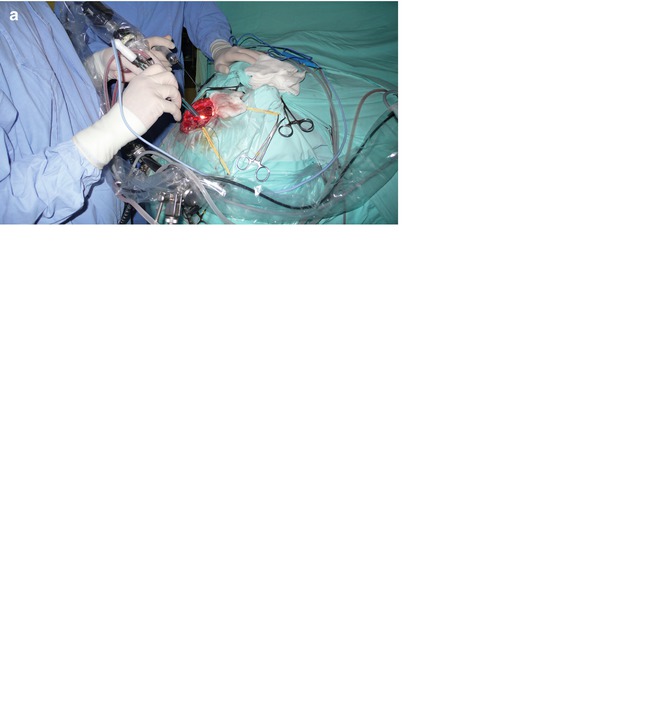
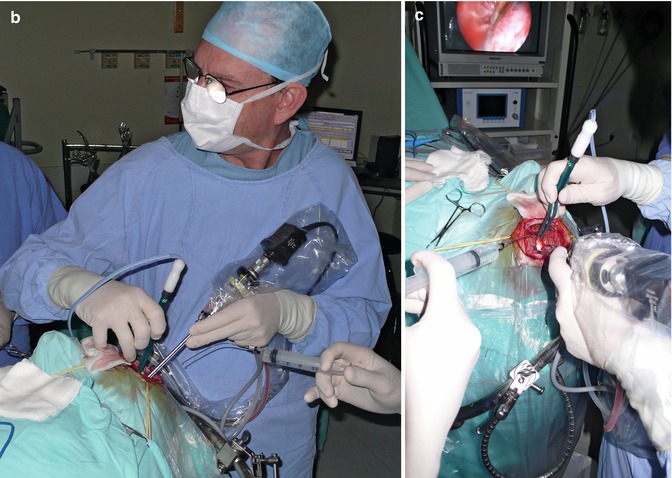
Fig. 13.10
(a, b, c) Intraoperative dissection work: bimanual dissecting. No need for brain retraction
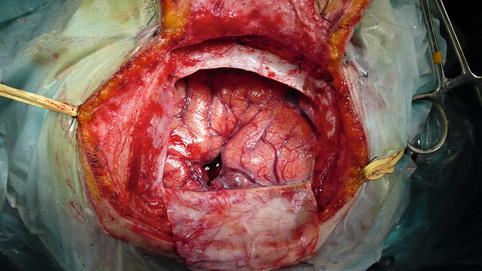
Fig. 13.11
Operating field at the end of surgery showing cerebral decompression and the 1 cm corticotomy needed for tumor removal. No brain retraction was necessary
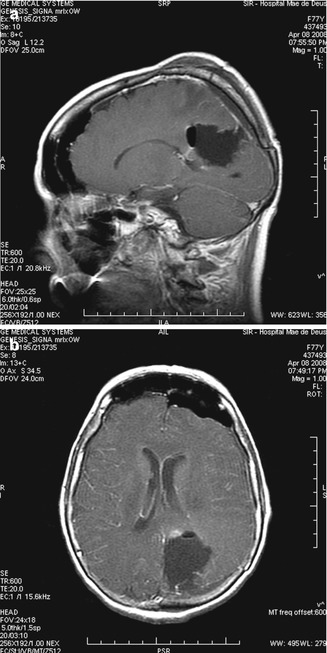
Fig. 13.12
(a, b) Immediate postop images. A larger craniotomy was performed for safety. Gross total removal was achieved
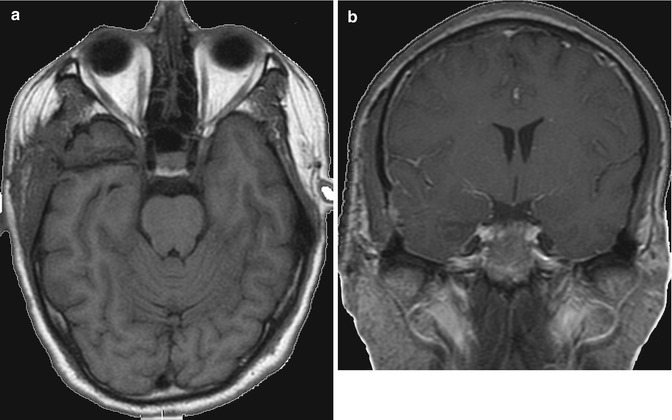
13.6.3 Cavernous Angioma
13.6.3.1 Case 1 (Fig.s. 13.13, 13.14, and 13.15)
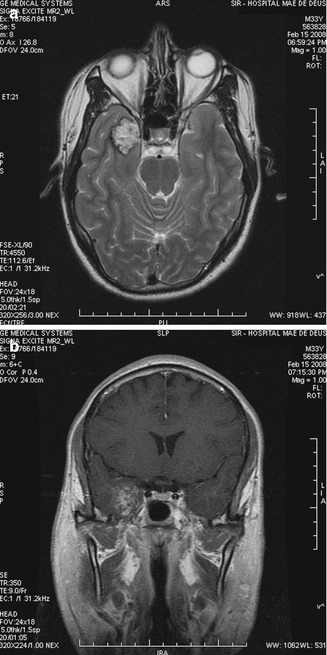
Fig. 13.13
(a, b) Temporomesial cavernous angioma. Preop images