CHAPTER 3
Neurohistology, Embryology, and Developmental Disorders
I. Neurohistology
A. Neurons: classified by the number of processes
1. Pseudounipolar: located in the spinal dorsal root ganglia and sensory ganglia of the cranial nerves V, VII, IX, X
2. Bipolar: found in the cochlear and vestibular ganglia of cranial nerve VIII, in the olfactory nerve, and in the retina
3. Multipolar: the largest population of nerve cells in the nervous system; includes the motor neurons, neurons of the autonomic nervous system, interneurons, pyramidal cells of the cerebral cortex, and Purkinje cells of the cerebellar cortex
B. Nissl substance: consists of rosettes of polysomes and rough endoplasmic reticulum; therefore, it has a role in protein synthesis; found in the nerve cell body (perikaryon) and dendrites and not in the axon hillock or axon
C. Axonal transport: mediates the intracellular distribution of secretory proteins, organelles, and cytoskeletal elements; inhibited by colchicine, which depolarizes microtubules
1. Fast anterograde axonal transport: responsible for transporting all newly synthesized membrane organelles (vesicles) and precursors of neurotransmitters; occurs at a rate of 200 to 400 mm per day; mediated by neurotubules and kinesin; neurotubule dependent
2. Slow anterograde transport: responsible for transporting fibrillar cytoskeletal and protoplasmic elements; occurs at a rate of 1 to 5 mm per day
3. Fast retrograde transport: returns used materials from the axon terminal to the cell body for degradation and recycling at a rate of 100 to 200 mm per day; transports nerve growth factor, neurotropic viruses, and toxins (e.g., herpes simplex, rabies, poliovirus, and tetanus toxin); mediated by neurotubules and dynein
D. Wallerian degeneration: anterograde degeneration characterized by the disappearance of axons and myelin sheaths and the secondary proliferation of Schwann cells; occurs in the central nervous system (CNS) and peripheral nervous system (PNS)
E. Chromatolysis: the result of retrograde degeneration in the neurons of the CNS and PNS; there is loss of Nissl substance after axotomy
 NB:
NB:
Axonal sprout grows at the rate of 3 mm per day in the PNS.
F. Glial cells: nonneural cells of the nervous system
1. Macroglia: consists of astrocytes and oligodendrocytes
a. Astrocytes: project foot processes that envelop the basement membrane of capillaries, neurons, and synapses; form the external and internal glial-limiting membranes of the CNS; play a role in the metabolism of certain neurotransmitters (e.g., γ-aminobutyric acid, serotonin, glutamate); buffer the potassium concentration of the extracellular space; form glial scars in damaged areas of the brain; contain glial fibrillary acidic protein, a marker for astrocytes; contain glutamine synthetase
b. Oligodendrocytes: myelin-forming cells of the CNS; one oligodendrocyte can myelinate up to 30 axons.
2. Microglia: arise from monocytes and function as the scavenger cells (phagocytes) of the CNS
3. Ependymal cells: ciliated cells that line the central canal and ventricles of the brain; also line the luminal surface of the choroid plexus; produce the cerebrospinal fluid
4. Tanycytes: modified ependymal cells that contract capillaries and neurons; mediate cellular transport between the ventricles and the neuropil; project to hypothalamic nuclei that regulate the release of gonadotropic hormone from the adenohypophysis
5. Schwann cells: derived from the neural crest; myelin-forming cells of the PNS; one Schwann cell can myelinate only one internode; separated from each other by the nodes of Ranvier.
G. Blood–brain barrier: consists of the tight junctions of nonfenestrated endothelial cells; some authorities include the astrocytic foot processes; although the blood-cerebrospinal fluid barrier consists of the tight junctions between the cuboidal epithelial cells of the choroid plexus, it is permeable to some circulating peptides (e.g., insulin) and plasma proteins (e.g., prealbumin).
 NB:
NB:
Areas of the brain that contain no blood-brain barrier include the subfornical organ, area postrema, and neurohypophysis.
H. Classification of nerve fibers
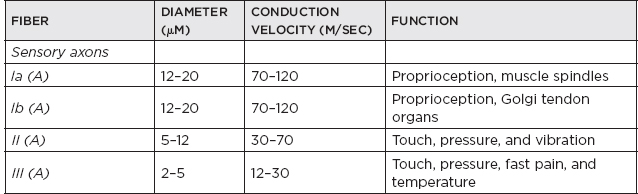
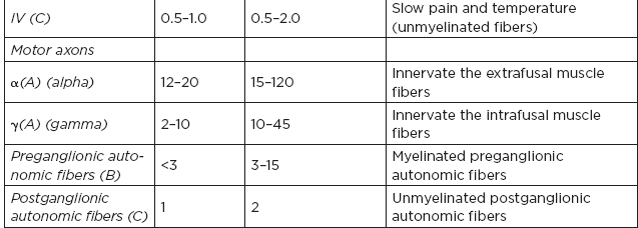
I. Cutaneous receptors
1. Free nerve endings: nociceptors (pain) and thermoreceptors (cold and heat)
2. Encapsulated endings: touch receptors (Meissner’s corpuscles) and pressure and vibration receptors (Pacinian corpuscles)
3. Merkel disks: unencapsulated light-touch receptors
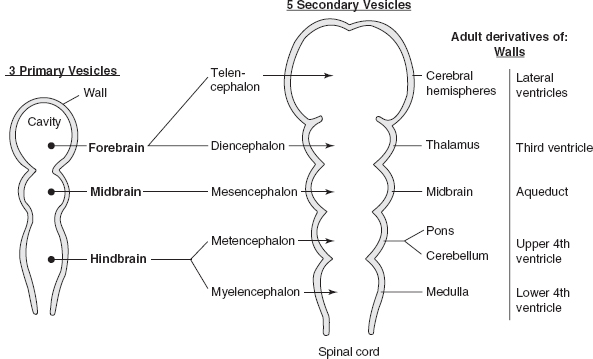
Figure 3.1 Embryologic derivatives of walls and cavities.
II. Embryology
A. NB: Ectoderm is the main embryonal layer forming the nervous system.
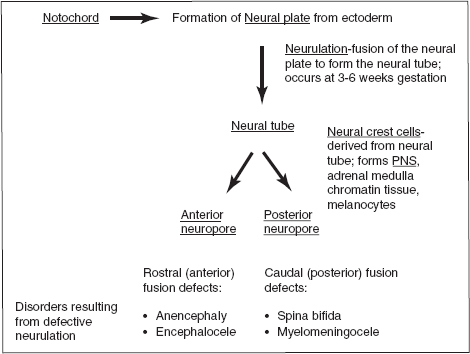
Figure 3.2 Neural tube formation.
B. Segmentation of neural tube
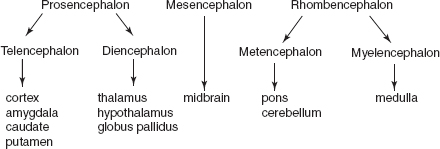
 NB:
NB:
Telencephalon produces the cerebral hemispheres and striatum except for the globus pallidus, which is from the diencephalon.
C. Sulcus limitans: marks boundary between basal and alar plates
1. Alar plate forms: posterior horn, gray matter, cerebellum, inferior olive, quadrigeminal plate, red nucleus, sensory brainstem nuclei
2. Basal plate forms: anterior horn, gray matter, motor nuclei of the cranial nerves
D. Cells derived from neural crest: chromaffin cells, preganglionic sympathetic neurons, dorsal root ganglia cells, skin melanocytes, adrenal medulla, cranial nerve sensory ganglia, autonomic ganglia, cells of pia/arachnoid, Schwann cells, odontoblasts (which elaborate predentin)
 NB:
NB:
The olfactory epithelium is from the ectoderm.
E. Neural tube formation
1. Closure of neural tube: begins at the region of 4th somite and proceeds in cranial and caudal directions; fusion begins on day 22
2. Anterior neuropore: closes on day 25
3. Posterior neuropore: closes on day 27
 NB:
NB:
α-Fetoprotein is found in the amniotic fluid and maternal serum; it is an indicator of neural tube defects (e.g., spina bifida, anencephaly); it is reduced in mothers of fetuses with Down syndrome.
F. Secondary neurulation (caudal neural tube formation): forms on days 28 to 32; forms the sacral/coccygeal segments, filum terminale, ventriculus terminalis
G. Neuronal proliferation: radial glia—earliest glia in embryonic CNS, provide guidance for neuron migration from ventricular region to cortex, may be precursors to astrocytes/oligodendrocytes but persist as Bergmann glia in mature cerebellum (which are special cerebellar cells whose processes extend to the pial surface)
1. Phase 1: between 2 and 4 months; neuronal proliferation and generation of radial glia
2. Phase 2: between 5 and 12 months; mostly glial multiplication
H. Neuronal migration: radial cells send foot processes from the ventricular surface to the pial surface, forming a limiting membrane at the pial surface; proliferate units of the ventricular zone migrate via the radial glia scaffolding to become the neuronal cell columns; the later migrating cells take a more superficial position (inside-out pattern). Types:
1. Radial: primary mechanism for formation of the cortex and deep nuclei, cerebellar Purkinje cells, and cerebellar nuclei
2. Tangential: originates in the germinal zones of the rhombic lip and migrate to form the external and internal granular layers
 NB:
NB:
All six layers of cerebral cortex are present at 27 weeks gestation (layer 1 is the most superficial):
1. Molecular layer
2. External granular layer
3. External pyramidal layer
4. Internal granular layer
5. Internal pyramidal layer
6. Multiform layer
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






