Clinical suspicion for Cushing’s syndrome
↓
Screening tests (first-line—perform at least 2)
• Urinary free cortisol (three 24 h collections)
• Low-dose (1 mg) overnight dexamethasone suppression
• Late-night salivary cortisol level
↓
Confirmatory testing
• Endocrinologist referral r/o physiologic hypercortisolism
• Confirmation of screening tests
• Second level testing if needed
1. Midnight plasma cortisol
2. Cortisol diurnal rhythm
3. 2 mg dexamethasone suppression test +/− CRH test
2.
CS etiology and differential diagnostic testing (Fig. 17.1): Appropriate therapy for CS requires an etiologic diagnosis between adrenal, pituitary, and an ectopic ACTH-producing tumor (EAS) [20, 21]. Understanding the tests used to establish the etiology requires knowledge of the hypothalamic–pituitary–adrenal (HPA) axis (see Fig. 17.1) [19, 22]. The first step to establishing the etiology is an ACTH level to determine ACTH dependency—either “independent” for adrenal nodule(s) or “dependent” for a CD or EAS (see Table 17.2) [23].
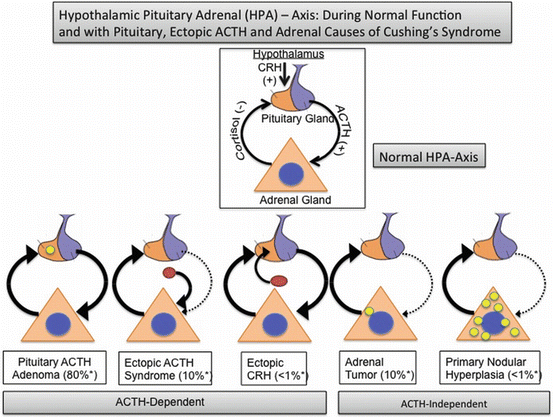

Fig. 17.1
Hypothalamic–pituitary–adrenal axis: during normal function and with various etiologies of Cushing’s syndrome
Table 17.2
Differential diagnostic testing in patients with proven Cushing’s syndrome
Test | Adrenal CS | Pituitary CS | Ectopic ACTH syndrome |
|---|---|---|---|
ACTH level | Low | Normal/high | Normal/very high |
CT/MRI adrenal g. | Mass(es) | Normal/hyperplasia | Normal/hyperplasia |
HDDSTa | No suppression | Suppression | Rare suppression |
CRH test | No response | Response | Rare response |
MRI pituitary | Normalb | Tumor (60 %) | Normalb |
IPSS | Not applicable | Gradient (pit./peripheral) | No gradient (pit./peripheral) |
(a)
ACTH-independent CS (see Table 17.2): Approximately 10 % of CS is ACTH-independent including adrenal tumors (both benign and malignant) and primary nodular hyperplasia. IPSS is not indicated in cases of ACTH-independent CS where an adrenal etiology is likely [24–26]. Adrenal CS can be easily diagnosed by the combination of low ACTH levels and finding adrenal nodule(s) by CT or MRI.
(b)
ACTH-dependent CS (see Tables 17.2 and 17.3): The vast majority of CS is ACTH dependent—largely from two causes: CD (~80 %) and EAS (~10 %) [27]. Many different tumor types both benign and malignant can produce EAS, and many produce other ectopic biomarkers (see Table 17.3) [5, 27–29]. Historically, differentiating CD from EAS had been difficult but that changed when IPSS was introduced [1, 30, 31]. Since then, it has achieved a high level of diagnostic accuracy. This has improved with modifications including bilateral sampling [32, 33], CRH stimulation, and “normalizing” ACTH responses with prolactin measurements. To demonstrate how IPSS is used in clinical practice, a case is presented below.
Table 17.3
Examples of hormonally active tumors associated with ectopic ACTH secretion
Source | Biomarker(s) |
|---|---|
Neuroendocrine/carcinoid tumors | 5-HIAA, chromogranin A, and serotonin |
Gastrinoma | Gastrin |
Medullary thyroid carcinoma | Calcitonin |
Pheochromocytoma | Catecholamines/metanephrines |
Bronchogenic carcinoma | Hypercalcemia |
Small cell lung carcinoma | ADH |
Pancreatic carcinoma | GHRH |
3.
Illustrative case 1: A 24-year-old woman with weight gain and Cushing’s features:
(a)
Clinical suspicion of CS: A 24-year-old woman experienced a 100-lb. weight gain with Cushing’s features, hyperglycemia, hypokalemia, hypertension, and psychoses requiring multiple psychiatric medications (Figs. 17.2 and 17.3). Her history was complicated by a series of undetermined quantities of Depo glucocorticoid injections over the last 2 years for back pain. Despite the history of exogenous glucocorticoids, CS testing was done because of the disproportionality of her findings. Exogenous CS was deemed an unlikely factor based on the rough assessment of dose, frequency, and remote history. In addition, normal cortisol responses to ACTH stimulation testing further diminished this possibility.
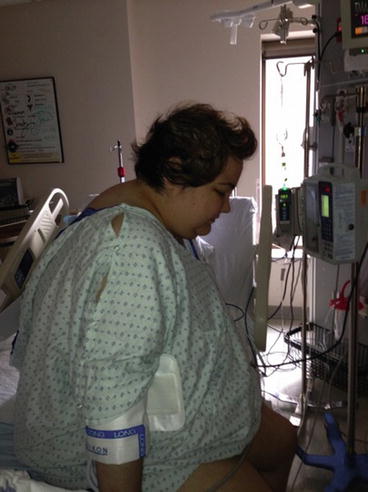
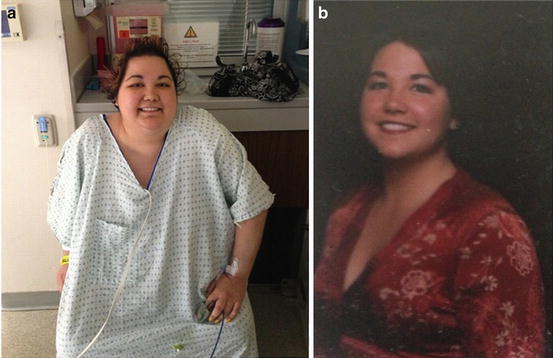

Fig. 17.2
A 24-year-old woman with Cushing’s syndrome taken during first hospitalization for diagnostic testing

Fig. 17.3
(a) A 24-year-old woman with Cushing’s syndrome taken during first hospitalization for diagnostic testing. (b) Same woman taken approximately 4 years earlier
(b)
Diagnostic CS testing: The diagnostic CS testing showed hypercortisolism based on late-night serum and salivary and 24-h urine cortisol values (see Table 17.4). Loss of the normal glucocorticoid feedback inhibition was found by dexamethasone suppression testing (DST). ACTH independence was excluded by non-suppressed plasma ACTH levels, thus ruling out adrenal CS.
Table 17.4
Diagnostic testing for Cushing’s syndrome in a 24-year-old woman with Cushing’s features
Date | DST (1 mg) 8 am Plasma cortisol | Plasma ACTH | 24-h urine free cortisol values | Late-night salivary cortisol | Late-night plasma cortisol |
|---|---|---|---|---|---|
Normal values | <2.0 μg/dl | 7–63 pg/ml | 0–50 μg/day | <0.112 μg/dl | <2.0 μg/dl |
4/19/2013 | 35a | 139a | |||
5/1/2013 | 22a | 0.365a | |||
5/11/2013 | 13.6a | ||||
5/12/2013 | 149a | 16.8a | |||
5/13/2013 | 169a | 25.9a | |||
5/14/2013 | |||||
5/15/2013 | 13.6a | 16.6a | |||
5/16/2013 | 14.6a | 29.1a | |||
7/22/2013 | 141a |
(c)
Differential diagnostic testing for CS etiology: Although an adrenal etiology of CS was ruled out by normal plasma ACTH levels (and later incidental abdominal CT imaging), the differentiation between CD and EAS was uncertain because of equivocal pituitary MRI findings and nondiagnostic hepatic mass pathology.
(i)
Ectopic ACTH syndrome evaluation: The patient’s abdominal CT scan showed multiple large masses most consistent with hepatic adenomas, which have not been associated with EAS. Consideration was given to hepatic carcinoid but the urinary 5-HIAA levels were normal.
(ii)
Pituitary MRI with gadolinium enhancement: Nondiagnostic findings suggested a small 2 × 4 mm left-sided adenoma (Fig. 17.4). The pituitary MRI was repeated with the same result. Because of the likelihood of CD and the accuracy and possible lateralization, we proceeded with IPSS.
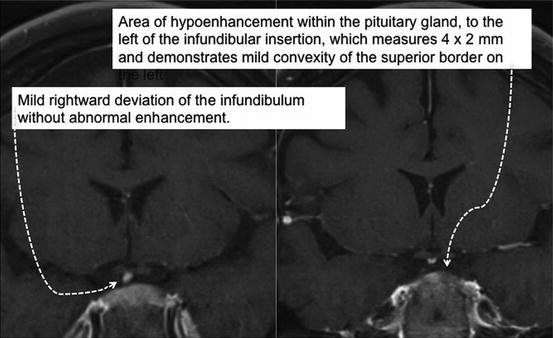

Fig. 17.4
Pituitary MRI findings in a 24-year-old woman with CS
(iii)
IPSS: Results of the IPSS angiography confirmed correct catheter placement (Fig. 17.5a, b). Increased ACTH production was localized to the pituitary gland confirming the diagnosis of CD (Tables 17.5 and 17.6). ACTH production within the pituitary body lateralized to the left side consistent with the MRI findings (Table 17.5).
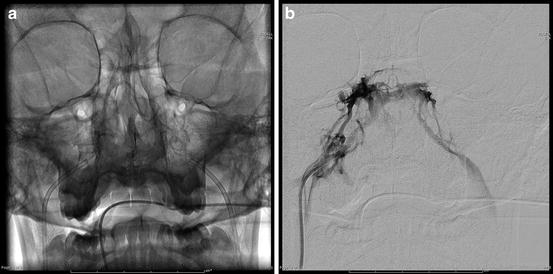

Fig. 17.5
(a) Fluoroscopic visualization of catheter placement in bilateral inferior petrosal sinuses. (b) Venogram of cavernous sinuses obtained from catheter in right inferior petrosal sinus
Table 17.5
IPSS testing in a 24-year-old woman with Cushing’s syndrome to localize and lateralize the source of ACTH
Condition | Baseline-1 | Baseline-1 | Baseline-2 | Baseline-2 | CRH+15 | CRH+15 | CRH+30 | CRH+30 |
|---|---|---|---|---|---|---|---|---|
Time | 11:54 AM | 11:54 AM | 11:59 AM | 11:59 AM | 12:14 PM | 12:14 PM | 12:29 PM | 12:29 PM |
Test | ACTH | Prolactin | ACTH | Prolactin | ACHT | Prolactin | ACTH | Prolactin |
Normal | 7–63 pg/ml | 4–23 ng/ml | 7–63 pg/ml | 4–23 ng/ml | 7–63 pg/ml | 4–23 ng/ml | 7–63 pg/ml | 4–23 ng/ml |
Left IPS | 648.9 | 58.7 | 540.5 | 57.2 | 1099 | 62.5 | 1,137 | 103.8 |
Right IPS | 631.8 | 124.8 | 460.8 | 73.6 | 823.7 | 145.3 | 648.3 | 167.5 |
Peripheral | 88.5 | 29.2 | 92.5 | 27.8 | 113.2 | 28.7 | 102.6 | 26.6 |
Table 17.6
Analysis of the IPSS testing in a 24-year-old woman with Cushing’s syndrome to localize and lateralize the source of ACTH
Localization of ACTH source | Lateralization of ACTH within the pituitary |
|---|---|
IPS:P ACTH basal (>2) = 6.6a | IPS ACTH L:R basal (>1.4) = 1.1 |
IPS:P ACTH CRH (>3) = 11.1a | IPS ACTH L:R CRH (>1.4) = 1.5a |
IPS:P PROL basal (>1.8) = 2.0a | IPS PROL L:R basal (~1) = 0.5 |
IPS:P PROL CRH (>1.8) = 3.9a | IPS PROL L:R CRH (~1) = 0.5 |
IPS:P ACTH/PROL (>0.8) = 2.8a | IPS ACTH/PROL L:R basal = 1.8a |
IPS ACTH/PROL L:R CRH = 3.0a |
(d)
Summary: This case summarizes the strategic use of IPSS in the medical evaluation of CS. Clinical suspicion to screen for CS was based on the rapidity and severity of her symptomatology disproportionate to her exogenous glucocorticoid exposure. The diagnosis of CS was made by multiple screening tests but the etiology was unclear because of equivocal pituitary MRI findings and the discovery of hepatic masses. Adrenal CS was unlikely based on non-suppressed plasma ACTH levels and normal adrenal gland imaging. On transsphenoidal surgery she was found to have a left-sided pituitary adenoma that was resected, following which the cortisol levels returned to normal.
4.
Diagnostic accuracy of IPSS in ACTH-dependent CS: As shown in this case, differentiating the etiology of CS between adrenal, pituitary, and EAS is essential for proper therapy. Adrenal CS has been relatively straightforward to diagnose with a combination of low ACTH levels and imaging showing adrenal mass(es) (see Table 17.2). Given the frequent small size of pituitary tumors and occasional occurrence of occult EAS tumors, differentiating CD and EAS can be difficult [34–37]. When IPSS has been used to confirm or exclude a pituitary source of ACTH in CS, it has been very successful with reports of diagnostic accuracy of around 90 % [38–43]. These tests are discussed below (see Table 17.2).
(a)
Plasma ACTH: This test will usually diagnose adrenal CS based on a low serum level (see Table 17.2). The combination of a low plasma ACTH with normal but autonomous urinary cortisol occurs in adrenal “incidentalomas.” CD usually has normal or high ACTH levels, whereas EAS has the highest but considerable overlap occurrence.
(b)
Adrenal gland CT or MRI: The combination of hypercortisolism with low ACTH should prompt adrenal imaging. The usual finding is adrenal mass(es) usually unilateral but sometimes bilateral. The finding of an adrenal mass in CS without a low ACTH level, however, is not an evidence for an adrenal etiology since these patients may still have CD [44].
(c)
High-dose dexamethasone suppression testing (HDDST): CD is based on the premise that most ACTH-secreting pituitary adenomas have an intact but relatively resistant negative feedback loop. In contrast, EAS has a negative feedback loop which is completely resistant to glucocorticoids with the exception of some carcinoid tumors. Unfortunately, using <50 % suppression “cut-point” yields false-positive and false-negative rates between 20 and 30 %, respectively [45].
(d)
CRH testing (Ferring Pharmaceuticals, Limhamn, Sweden, under the tradename Acthrel): CRH can be difficult to procure for outpatient testing but it is considered second best for differentiating CD and EAS. The fact that most ACTH-secreting pituitary adenomas (85 %) respond to CRH with an increase in ACTH secretion and most EAS and adrenal CS do not is the basis for this test [46]. Dexamethasone is sometimes used in conjunction with CRH to improve the diagnostic accuracy, but the test has enough false-negative results to limit its use. The major use for this test is diagnosing pseudo-Cushing’s and physiologic hypercortisolemic states (see Table 17.2) [47].
(e)
Pituitary MRI (with gadolinium enhancement): Pituitary MRI has a diagnostic accuracy of only about 50 % largely as a result of two problems [48–51]. First, the small size of CD adenomas (sometimes ~1 mm) and second, lack of functional assessment, which is a problem with non-ACTH-secreting pituitary “incidentalomas.” Pituitary incidentalomas have been found in 10–20 % of unselected MRIs and autopsies [52–54]. Co-lateralization of MRI findings with IPSS will further enhance not only diagnostic certainty but also lateralization within the pituitary body [55, 56].
(f)
IPSS (90 % diagnostic accuracy): Of all of the etiologic tests for CS, IPSS has the best diagnostic accuracy as reported by high-volume referral centers [57]. The primary use of IPSS is to localize the source of ACTH (see Table 17.7). The secondary and less accurate use of IPSS is to lateralize the side of ACTH source within the pituitary body to allow for more selective and less invasive transsphenoidal surgery. It must be remembered that IPSS is not useful in diagnosing CS. IPSS has proven useful in both pregnancy [58] and young children [17, 59–61]. The diagnostic IPSS criteria to localize and lateralize the source of ACTH are seen in Table 17.8. Despite its successes IPSS still has inaccuracies in otherwise difficult cases [62, 63]. Furthermore, because IPSS requires a high degree of technical skill and expertise, results could vary widely. Anatomic variability precludes about 10 % of CS patients who cannot be successfully catheterized for IPSS. The principal reason for CRH stimulation is to correct for ACTH pulsatility [64]. Pulsatility is also a good reason to draw two baseline ACTH levels. CRH stimulation improves IPSS diagnostic accuracy from 60 to 90 % according to several reports [56, 65, 66]. A systematic analysis that included 21 studies and 569 patients found that IPSS with CRH stimulation achieved 96 % sensitivity and 100 % specificity in discriminating Cushing’s disease from EAS [47]. Interestingly, CRH also stimulates AVP, prolactin, and other ACTH-related peptides [67–69]. CRH has also been combined with TRH (not commercially available) to dually stimulate both ACTH and prolactin [70]. Difficulty procuring CRH has led to the interest in alternatives such as naloxone, metyrapone, and vasopressin [71, 72]. A synthetic version of vasopressin and desmopressin (DDAVP; Ferring Pharmaceuticals) compared to CRH produces less ACTH stimulation [73–75]. Nevertheless, desmopressin stimulation improves diagnostic accuracy over 90 % and comparable to CRH [76]. Combined CRH and AVP stimulation also yields excellent diagnostic accuracy and perhaps better than either alone [77, 78]. The desmopressin technique is similar to CRH and 10 mg of desmopressin is used in place of the CRH. IPSS prolactin levels can be used to correct for venous effluent concentrations as they lateralize in the majority of non-CS subjects showing the need to correct for difference in venous effluent [79–84]. When petrosal-to-peripheral ACTH ratios are “normalized” to prolactin petrosal-to-peripheral ratios, the diagnostic accuracy for both localization and lateralization improves [82, 85]. “Normalization” of ACTH gradients improves accuracy not only prospectively but retrospectively using archived IPSS blood samples [79, 84]. In the current case, ACTH gradients lateralized to the left (Table 17.6) only after prolactin normalization, which is where the CD adenoma was found. Beyond prolactin, other IPSS neuroendocrine biomarkers and pituitary hormones have yet to become useful [69, 86, 87]. IPSS lateralization refers to determining the side, either right or left, of the CD adenoma within the pituitary body. Lateralization unfortunately has not achieved the diagnostic accuracy of localizing the source of ACTH, either within the pituitary body or not. Some studies have shown that a gradient of ≥1.4 between the ACTH concentrations in the two sinuses predicted the side of the tumor with up to 71 % accuracy if catheters were appropriately placed [48, 49, 88–92]. However, others have not found such strong predictive values. Since there is a 50 % chance of correctly predicting the location without the aid of any anatomical data, this expensive procedure cannot be justified for lateralization alone [93–95]. If MRI lateralizes a pituitary lesion to the same side, the accuracy is much higher when it does not. If the IPPS lateralizes but the MRI does not, the whole pituitary body should still be explored at TSS since ~30 % have contralateral tumors [91]. The reasons for failing to lateralize are listed in Table 17.9. Besides the usual considerations of variations in venous drainage and catheter placement, an intrinsic lateralization of pituitary function favors the right of the time 80 %. This may explain why left-sided IPSS lateralization is most accurate for CD. Other causes are seen in the table and should be considered when evaluating the data.
Table 17.7
Utility of inferior petrosal sinus sampling (IPSS)
Primary |
Differentiate the cause of Cushing’s syndrome, i.e., pituitary disease versus ectopic ACTH |
Secondary (less helpful) |
Pituitary lateralization |
Not helpful |
Establishing diagnosis of endogenous CS |
Table 17.8
IPSS criteria to localize and lateralize the source of ACTH
Purpose | Catheter | Test | Condition | Criteria |
|---|---|---|---|---|
Localize ACTH | IPS:P | ACTH | Pre-CRH stim | >2 |
Localize ACTH | IPS:P | ACTH | Peak-CRH stim | >3 |
Catheter placement | IPS:P | Prolactin | Pre- or peak-CRH | >1.8 |
Excess ACTH | IPS:P | ACTH:prolactin | Pre-CRH stim | >0.8 |
Lateralize ACTH | IPS L:R | ACTH | Pre- or peak-CRH | >1.4 |
Lateralize prolactin | IPS L:R | Prolactin | Pre- or peak-CRH | ~1.0 |
Corrected ACTH lateralization | IPS L:R | ACTH:prolactin | Pre- or peak-CRH | >1.4 |
Table 17.9
Some rare difficulties in IPSS’ ability to localize the source of ACTH (CD vs. EAS) and to lateralize CD within the pituitary
Failure to localize (pituitary vs. EAS) False (+) ~5 %; false (−) ~15 % | Failure to lateralize (right vs. left) |
|---|---|
1. Anomalous venous drainage | 1. Anomalous venous drainage |
2. Incorrect catheter placement | 2. Incorrect catheter placement |
3. Ectopic CRH-secreting tumor (carcinoid) | 3. Ectopic tumor secreting CRH |
4. Cyclic hypercortisolism | 4. Prior transsphenoidal surgery |
5. Inadequate examination of surgical pathology | 5. Inadequate examination of surgical pathology |
6. Extrapituitary parasellar ACTH-secreting adenoma | 6. Extrapituitary parasellar ACTH-secreting adenoma |
7. Failure to employ CRH stimulation | 7. Midline microadenoma |
8. Inability to catheterize | 8. Sample withdrawal was too rapid |
9. Medical or surgical therapy: to decrease adrenal cortisol and/or pituitary ACTH secretion | 9. Intrinsic functional laterality of hypothalamic–pituitary axis (80 % right-side dominant) |
10. Failure to diagnose CS with certainty and exclude physiologic hypercortisolism | 10. Mislabeling left and right sample tubes |
(i)
Get Clinical Tree app for offline access

Accuracy of IPSS localization—false positives: A false-positive IPSS occurs when the ACTH source is localized to the pituitary body, but TSS neither finds the adenoma nor is curative. The IPSS false-positive rate is very low and in some studies approaching 0 %. Some causes of false-positive IPSS localization (as well as lateralization) results are seen in Table 17.9.
(1)
Normals and pseudo-Cushing’s states: The most important step to avoid a false-positive IPSS is to be certain of the diagnosis of CS. IPSS in normal subjects will localize ACTH secretion from the pituitary body. Therefore, IPSS is not helpful in confirming or establishing the diagnosis of CS only in its etiology. Particular concern occurs in pseudo-Cushing’s states which can biochemically and/or clinically mimic true CS. In some of these conditions, all of the usual screening and diagnostic tests for CS will be positive [96, 97].
(2)
Extrapituitary parasellar ACTH-secreting adenomas: One rare cause of a positive IPSS but negative pituitary surgery is an extrapituitary, parasellar ACTH-secreting (ectopic) adenoma [98, 99]. This occurs in ~1 % of CD cases and is sometimes associated with an empty sella. The extrapituitary adenomas probably develop from a remnant of Rathke’s pouch, which failed to migrate to the anterior pituitary. The extrapituitary adenomas have been found in the sphenoid sinus, anterior cranial fossa, interpeduncular cistern, intraclival, and suprasellar locations. In most instances, careful examination of MRI images will reveal their location. It is important to consider the possibility of an extrapituitary adenoma especially if EAS is not found and before committing the patient to a total hypophysectomy and/or bilateral adrenalectomy [99–101].
(3)
Ectopic CRH: Although very rare, ectopic tumors secreting CRH, especially bronchial carcinoids, will produce “eutopic” ACTH and cause IPSS localization to the pituitary body. Inhibiting adrenal corticosteroidogenesis (medical or surgical) in EAS will potentially restore pituitary ACTH secretion. Combined ectopic ACTH and CRH production has also been reported to give false-positive IPSS results [102].
(4)




MRI negative: If IPSS is positive for a gradient (pituitary: peripheral) but the pituitary MRI is negative, TSS success drops almost in half (98–50 % in one study). In those unsuccessful cases, no pituitary tumor has subsequently been found in a 4-year follow-up suggesting an occult pituitary tumor was not missed [56, 103].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








