Fig. 15.1
Retinal and subhyaloid hemorrhage in a patient with Terson syndrome as result of a ruptured middle cerebral artery aneurysm. Note typical appearance of triangular-shaped hemorrhage to subhyaloid space in inferior retina
The intimate relationship between the anterior visual pathways and the circle of Willis makes visual loss a frequent presenting symptom of cerebral aneurysms. Cerebral aneurysms arising from intracranial carotid artery, middle cerebral artery, and anterior communicating arteries are more likely associated with visual loss than aneurysms arising from basilar and posterior communicating aneurysms [9]. Involvement of the optic nerve gives rise to decreased central vision and optic atrophy. Compression of the optic chiasm results in bitemporal field defects. Aneurysms growing posteriorly may cause homonymous hemianopia from involvement of the optic tract. Ophthalmic and supraclinoid segment carotid aneurysms constitute less than 10 % of all cerebral aneurysms [6, 16]. They may present with headache, orbital pain, and visual loss by compression and without rupture. Middle cerebral artery and anterior communicating artery aneurysms account for nearly half of all cerebral aneurysms. They tend to rupture and present with subarachnoid hemorrhage before enlarging sufficiently to affect visual function [17].
Diplopia and ocular motility abnormalities are commonly associated with aneurysms arising from carotid-cavernous segment, posterior communicating, and basilar arteries [18]. Posterior communicating artery aneurysm is a frequent cause of third nerve palsy, which presents with a symptom complex of ptosis, fixed and dilated pupil, and the so-called down and out eye [19]. The pupil reactivity to light stimulus should be meticulously examined to help differentiate aneurysmal compression from an ischemic third nerve palsy. A complete third nerve palsy that spares the pupil is due to an ischemic third nerve palsy, while pupil involvement in combination with either a complete or an incomplete third nerve palsy raises a high suspicion for aneurysmal compression. An incomplete third nerve palsy without pupil involvement warrants close observation over the following 1 week for development of pupil abnormalities. Aneurysms within the cavernous sinus can affect the multiple cranial nerves that travel through cavernous sinus; the ocular motor abnormalities are sometimes associated with decreased sensation or pain along the V1 and V2 distributions of the trigeminal nerve [10]. Carotid-cavernous aneurysm may occasionally present with a sixth nerve palsy in combination with Horner syndrome, localizing the lesion to the cavernous sinus. Basilar aneurysms, while less common, constitute the majority of the cerebral aneurysms arising within the posterior fossa. Basilar artery aneurysms may cause diplopia via third, fourth, and sixth nerve involvement, skew deviation, and gaze palsy secondary to aneurysmal compression of the midbrain and pons. Basilar artery aneurysms can also cause homonymous visual field loss due to thromboembolic infarction to the occipital lobe [18].
The radiographic features and endovascular intervention for cerebral aneurysm are described in Chaps. 10 and 11.
Carotid-Cavernous Fistula
Introduction
The cavernous sinuses are a pair of cerebral venous sinuses located at the center of the skull base lateral to each side of the sella turcica (Fig. 15.2). They are bordered by the sphenoid and temporal bones. The cavernous sinus collects venous blood drained from the eye and orbit through the superior and inferior ophthalmic veins and then drains posteriorly to the internal jugular vein through the superior and inferior petrosal sinuses and the transverse sinus [20]. A number of cranial nerves and intracranial vessels travel through the cavernous sinus. The cavernous segment of the internal carotid artery is located in the medial aspect of the cavernous sinus and is surrounded by oculosympathetic fibers that form a fine plexus. Immediately lateral to the internal carotid artery is the sixth cranial nerve. The third and fourth cranial nerves and the first and second divisions of the trigeminal nerve (ophthalmic and maxillary nerve, respectively) travel along the lateral border of the cavernous sinus. The pituitary gland is located in the sella turcica between the pair of cavernous sinuses.
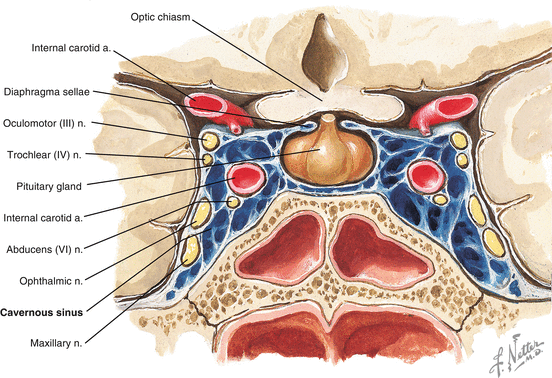

Fig. 15.2
Coronal view of the cavernous sinus demonstrating the passage of the third, fourth, fifth, and sixth cranial nerves and internal carotid artery inside the cavernous sinuses (permission from Netter’s production)
Carotid-cavernous fistula (CCF) is an abnormal communication between the cavernous sinus and the carotid arterial system. Meningeal branches arising from the internal carotid artery, external carotid artery, or both supply the dural sheath and anatomical structures contained in the cavernous sinus. When there is a breach in either the main trunk of the cavernous segment of the internal carotid artery or the meningeal branches from the internal or external carotid artery, an abnormal communication develops between the arterial and venous circulation [21]. Communication between the arterial and venous system results in elevated venous pressure thus elevated venous outflow resistance. Functional obstruction of the venous drainage from the eye and orbit ensues. The classification of CCF is based on anatomy (direct vs. indirect), cause (traumatic vs. spontaneous), or hemodynamic status (high flow vs. low flow). Each type of CCF is associated with specific clinical manifestations, treatment strategies, and outcomes. The most commonly used dichotomies in CCF classification are (1) direct CCF formed by direct connection between the cavernous segment of the internal carotid artery and cavernous sinus and (2) indirect CCF caused by a communication between the branches of the internal or external carotid arteries and the cavernous sinus.
Clinical Manifestation
A direct CCF is caused by a tear in the intra-cavernous segment of the internal carotid artery, usually in the setting of trauma, although in a small proportion of patients, it may occur spontaneously [22–26]. Direct CCF tends to affect younger patients and men are affected more than women, reflecting the higher incidence of trauma in these groups. The classic triad of proptosis, conjunctival chemosis, and orbital bruit is consequence of significantly elevated venous pressure in the superior ophthalmic vein and cavernous sinus system [26–28]. Typical ocular findings are prominent proptosis, chemosis, hyperemia, and irritating periorbital pain or headache. Elevated intraocular pressure and secondary glaucoma are caused by increased episcleral pressure and vortex venous pressure, anterior shift of the lens-iris diaphragm, as well as neovascular glaucoma secondary to ocular ischemia [24]. Ophthalmoplegia can be a consequence of either edema of the extraocular muscles or damage to the cranial nerves as they travel through the cavernous sinus. Vision loss is common and is usually severe in direct CCF, caused by exposure keratopathy, glaucoma, ischemia of the optic nerve, or coexisting traumatic optic neuropathy [29].
In contrast to direct CCF, indirect CCFs usually have a spontaneous onset and are slowly progressive. A history of minor trauma is reported in a small group of patients. When compared to direct CCF, indirect CCF tends to occur in an older age group (mean from 50 to 69 years of age), and women constitute 60–90 % of all indirect CCF cases. Vascular changes in a variety of systemic conditions such as postmenopausal hormonal changes, pregnancy, hypertension, and atherosclerosis are hypothesized to predispose patients to the development of indirect CCFs [27, 28, 30, 31].
Clinical manifestations of indirect CCFs include red eye, discomfort, ocular hypertension, or diplopia. Eye findings include engorged, “corkscrew” episcleral vessels from arterialized venous blood, ocular hypertension, and ophthalmoparesis. Less common presentations of indirect CCFs include headache, pulse-synchronous tinnitus, vision loss, and venous retinopathy [26, 27, 30, 32–47]. When indirect CCFs drain posteriorly to the superior or inferior petrosal sinuses, they may be asymptomatic or manifest as isolated cranial nerve palsies; symptoms and signs of orbital congestion become noticeable when indirect CCFs change its drainage from posterior to anterior draining indirect CCFs [48–51].
Importantly, significantly elevated venous pressure in the cavernous sinus may be transmitted retrograde to the cortical veins (cortical venous drainage), resulting in hemorrhagic venous infarction. Cortical venous drainage may lead to severe neurological dysfunction such as hemimotor or hemisensory deficits, necessitating prompt intervention to close the arterial venous shunt. Among various clinical manifestations, the presence of bilateral orbital signs and a postauricular bruit was found to have the most predictive value of cortical venous drainage [52].
Radiographic Features
The most prominent radiographic feature of direct CCF and indirect CCFs on computed tomography (CT) or magnetic resonance imaging (MRI) is a dilated superior ophthalmic vein, although enlargement of the EOMs, abnormal cavernous sinus flow voids, and sometimes engorgement of the cavernous sinus with a convexity of the lateral wall can also be observed [53, 54]. Orbital ultrasonography may also provide sensitive and reliable measurement by demonstrating dilatation of the superior ophthalmic vein and enlargement of the EOMs [55]. A high index of suspicion should be maintained in patients with symptomatology as above and a prompt imaging study of the brain and orbit using CT or magnetic resonance tomography done for screening. Catheter angiography remains the only definitive study to confirm or eliminate the diagnosis. Dural AVFs can be very difficult to diagnose with noninvasive imaging and sometimes is recognized only on catheter angiography.
Management of CCF
Spontaneous resolution of indirect CCFs has been reported in 20–60 % of indirect CCFs in the literature [28, 31, 37, 39, 56]. A nonsurgical management of indirect CCFs is carotid-jugular compression. The compression entails intermittent, seconds to a few minutes of compression of the ipsilateral cervical carotid artery and internal jugular vein using the contralateral hand for a period of a few weeks to a couple of months [26, 39, 57]. This maneuver should be considered in patients whose symptoms are too mild to warrant immediate surgical intervention or in those whose age or systemic comorbidities predispose them to higher surgical complications.
Endovascular Treatment
Although a conservative approach has been advocated for direct carotid-cavernous fistulas when there is no rapidly progressive visual deterioration or cerebral ischemia, intermittent compression of the jugular and carotid artery is very unlikely to be effective. Resolution without recurrence has been described in only 17 % of attempted cases [57].
Conversely, indirect carotid-cavernous fistulas commonly develop insidiously, and a conservative approach might be appropriate. Approximately 30–36 % of indirect (or “dural”) CCFs may heal with conservative treatment utilizing manual external compression of the carotid artery and jugular vein [57, 58]. Close follow-up may be indicated to evaluate for the development of cortical venous drainage (which is correlated with higher risk of development of hemorrhagic complications or venous infarcts) [59]. Indications for treatment include persistently elevated intraocular pressure, visual deterioration, malignant proptosis, symptomatic ocular deviation, exposure keratopathy, severe pain, intolerable bruit, and/or diplopia.
Endovascular intervention has replaced intracranial surgery and is the treatment of choice when intervention is indicated. Endovascular treatment is typically the first-line approach for direct carotid-cavernous fistulas [60]. Compared to carotid artery surgery (trapping or ligation), endovascular intervention has significant lower risk of complications. The objective of the endovascular treatment is to eliminate the arteriovenous carotid-cavernous shunting. This leads to normalization of the venous pressures, reversing the ophthalmic and leptomeningeal venous retrograde flow and engorgement, as well as symptoms related to vascular steal.
The evaluation for feeding pedicles during catheter-based angiography is made through bilateral common, internal, and external carotid artery iodinated contrast injections. The endovascular therapy aiming to obliterate the fistulous connections may be performed through either trans-arterial or transvenous routes, and it is based on the arterial and venous angioarchitecture and flow patterns.
In indirect CCFs, trans-arterial embolization is technically difficult due to the small size and multiplicity of feeders. Therefore, transvenous embolization of CCF is the preferred approach for indirect CCFs. The cure rates have been reported between 70 and 90 %, with complication rates ranging between 2.3 and 5 % [60]. Technically, central venous access (femoral vein or internal jugular puncture) is obtained and followed by the introduction of a short vascular sheath. A 6-French guide catheter is introduced commonly through the common femoral vein and navigated into the distal internal jugular vein (sometimes directly into the internal jugular vein) and then into the jugular bulb, where a retrograde venogram is performed. The inferior petrosal sinus (IPS) may opacify and will constitute the path to reach the cavernous sinus. If the fistulous flow is high, the retrograde venogram may not opacify the IPS or any other cavernous sinus draining vein (such as the superior petrosal sinus). Therefore, a second diagnostic catheter (via a groin arterial puncture) is positioned in the common carotid artery ipsilateral to the side where the main arteriovenous shunting is present. This allows delineation of the venous outlets, which will fill early in the arterial phase, and guides the catheterization of the cavernous sinus under overlay technique or venous-phase road mapping. It also allows intermittent arterial runs to evaluate for resolution of the arteriovenous shunting during embolization. As stated, the cavernous sinus is most commonly accessed through the IPS, utilizing a microcatheter that is navigated from the catheter previously positioned in the jugular bulb. However, at times the IPS may be either thrombosed or composed of a plexiform structure. The contralateral IPS can be cannulated and the microcatheter navigated through the contralateral cavernous sinus, across the intercavernous sinus, and into the targeted side. Other endovenous route options are available and include catheterization of the superior petrosal sinus or the facial vein takeoff from the internal jugular vein (which leads to the angular vein, and then into the superior ophthalmic vein, and finally into the cavernous sinus).
If the aforementioned routes are technically challenging, the arterialized superior ophthalmic vein (if dilated) can be directly accessed via an eyelid or eyebrow incision, with subsequent dissection of the orbicularis oculi muscle, orbital septum, and fat until an arterialized venous branch is noted. The branch is followed proximally until the main trunk is found. A small incision is made, a J wire is inserted, and a 4-French pediatric short sheath (or a micropuncture sheath) is advanced. A gentle intraoperative angiogram is performed through the sheath to confirm the proper positioning [61].
Another, less studied, option involves percutaneous transorbital puncture leading to the catheterization of the superior or inferior ophthalmic veins or the cavernous sinus directly [61].
Regardless of the access route, the technique, the microcatheter is optimally positioned within the cavernous sinus (typically more anteriorly, close to the proximal aspect of the superior ophthalmic vein) and embolization can ensue. Commonly used options are coiling and/or embolization with ethylene vinyl alcohol copolymer (Onyx). The sinus is filled with coils and/or liquid embolics until there is complete distribution of the embolic material within the cavernous cavity, and no early venous drainage is observed through arterial runs. Ocular symptoms tend to improve over the following hours. Paradoxical worsening of the symptoms has been described by tend to be transient [62]. Coil overpacking or direct liquid embolic local effects on nerves may generate posttreatment cranial nerve deficits (which commonly improve) [43]. Reported endovascular cure rates for indirect CCFs range around 70–90 %, with complication rates of 2.3–5 % [41, 60]. Radiosurgery has been demonstrated in small series to be effective; however, the latency period for the obliteration of the fistula is typically of several months. It may have an important role for incompletely treated indirect fistulas [60, 63].
In direct CCFs, trans-arterial embolization with different materials is generally needed. A guide catheter is advanced into the ICA, and a microcatheter navigated from the arterial side navigated across the cavernous carotid arterial tear into the cavernous sinus. We favor filling of the cavernous sinus initially with coils in order to slow flow. Subsequently, ethylene vinyl alcohol copolymer (or N-butyl cyanoacrylate) may be injected to fill the remaining space. The major concern is that once the embolic material starts filling the cavernous sinus—which surrounds the ICA—visualization of the tear becomes difficult, and protrusion of the coils or regurgitation of embolic agent from the cavernous sinus back into the ICA may occur inadvertently. Therefore, extreme caution is required. Some operators advocate inflation of a compliant balloon in the ICA across the tear during embolization to avoid unintentional ICA embolization. Transvenous embolization may be performed; however, it is not preferred due to the risks of herniation of embolic material into the ICA. For direct CCFs, overall endovascular occlusion rates have been reported to be between 55 and 99 %, and the morbidity was described to be as high as 10–40 % [60]. Newer but less studied techniques include covered stents that obliterate the fistula through a direct physical barrier [60, 64].
Illustrative Case 1
A 60-year-old man presented with chemosis and increased intraocular pressures involving the left eye. Conventional angiography confirmed an indirect CCF, fed by bilateral internal and external carotid arteries (Fig. 15.3a, b). The cavernous sinus and the arterialized superior ophthalmic veins were clearly demonstrated (Fig. 15.3b). Although the inferior petrosal sinuses could not be visualized, the facial vein was clearly delineated (Fig. 15.3c). The microcatheter was advanced into the cavernous sinus through the facial vein (Fig. 15.3d), and Onyx was carefully injected. The left cavernous sinus was filled with the liquid embolic, and the fistulous point obliterated (Fig. 15.3e, f).
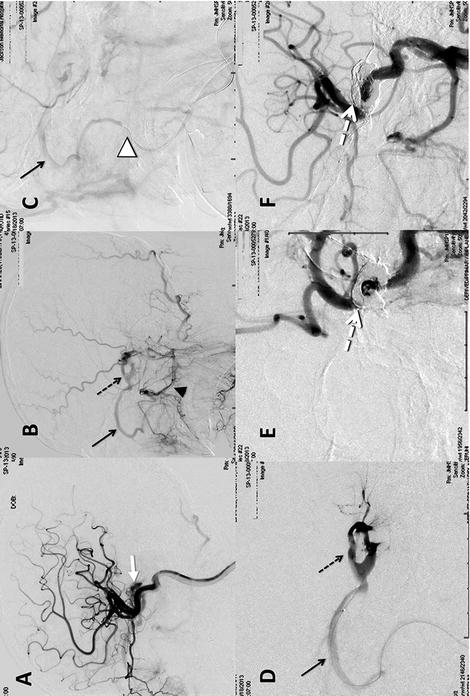

Fig. 15.3
(a) Lateral view of the left internal carotid artery injection revealing cavernous sinus early filling through the meningohypophyseal artery (white arrow). (b) Lateral view/mid-arterial phase left external carotid injection revealing internal maxillary (arrowhead) and ascending pharyngeal artery filling of the cavernous sinus (dotted arrow) and superior ophthalmic vein (arrow). (c) Lateral/late-arterial phase left external carotid injection revealing the microwire within the facial vein (white arrowhead). (d) Magnified lateral cavernous sinus (dotted arrow) venogram through the microcatheter. (e, f) AP and lateral final internal carotid angiograms revealing the Onyx cast (dotted white arrow) and no evidence of early venous drainage
Idiopathic Intracranial Hypertension
Introduction
Idiopathic intracranial hypertension (IIH), also called pseudotumor cerebri or benign intracranial hypertension, refers to a condition of elevated intracranial pressure unrelated to a space-occupying lesion, cerebral venous thrombosis, meningitis, or hydrocephalus. IIH has a predilection for obese women of child-bearing age, although it can occur in children, at older age, and in males [65–68]. IIH has been associated with a variety of medications including antibiotics (tetracycline, minocycline, doxycycline, and nalidixic acid), growth hormone, lithium, retinoids (both topical and oral), Lupron, Norplant, and cyclosporine. Obstructive sleep apnea and recent weight gain may also contribute to an elevated intracranial pressure [69].
A number of mechanisms are thought to contribute to the development of IIH, including increased cerebrospinal fluid production, reduced cerebrospinal fluid absorption, the influence of hormones, abnormal vitamin A metabolism, as well as elevated cerebral venous pressure. The role of elevated intracranial dural venous pressure in the pathophysiology of IIH has gained increasing attention, as a potentially treatable cause of IIH. Although stenosis of the transverse and sigmoid sinus is a common radiographic finding in IIH [70], it is unclear whether dural sinus stenosis is a cause of elevated intracranial pressure or a consequence of chronic compression of dural venous sinuses from persistently elevated intracranial pressure. Regardless of the etiology, increased resistance in cerebral venous outflow seems to be the common final pathway in the pathophysiology of IIH, suggested by elevated manometry measurements of the pre-stenotic vs. poststenotic pressure gradient [71–83].
Clinical Manifestation
The typical presentation of IIH includes headache, pulse-synchronous tinnitus, with varying degrees of vision loss, and papilledema. Headache occurs in about 90 % of IIH patients [84]. Pain in a nerve root distribution or retro-ocular pain with eye movement was found to be more specific, while all types of headaches can be seen [85, 86]. Pulse-synchronous tinnitus, described as a “whooshing” sound synchronized with the heart beat, is more specific for IIH if present. Patients may complain of transient visual obscurations and episodic and severe vision loss in both eyes lasting for seconds with complete recovery usually associated with activities that increase central venous pressure (such as Valsalva maneuver) or decrease systemic perfusion pressure (transition from supine or sitting to the upright position). Papilledema, manifested as hyperemia and elevation of the optic disc, engorgement of the central retinal veins, and obscuration of the central retinal vessels on the disc often with disc hemorrhage and exudates, is usually bilateral and symmetrical (Fig. 15.4). Most patients with IIH have mild vision loss that is reversible after appropriate treatment, although permanent vision loss can occur in about 25 % of patients [86]. A small proportion of patients (2–3 %) with IIH present with fulminant visual loss over days [87], necessitating aggressive intervention to salvage vision.
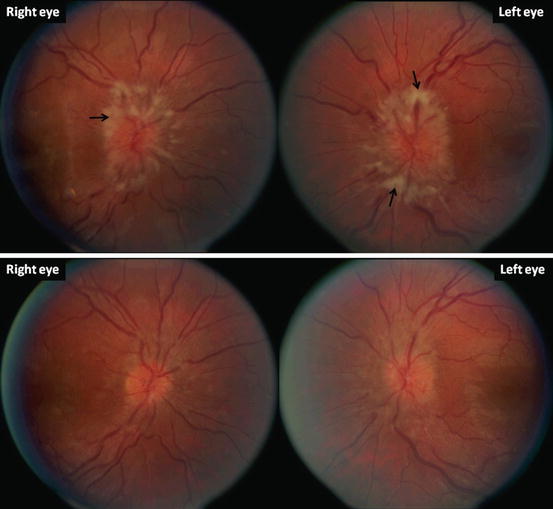

Fig. 15.4
Bilateral papilledema in a 26-year-old lady with a concurrent right sixth nerve palsy. Note the elevation of the optic disc, engorgement of the central retinal veins, and obscuration of the retina vessels both on the disc and as the vessels exit the disc margin (top). There are disc hemorrhages in the left eye. There are nerve fiber layer infarcts reflected by the white areas (arrows). The patient’s visual acuity was 20/40 in the right eye and 20/60 in the left eye. The patient had been on oral minocycline for 2 months for acne treatment prior to presentation. Minocycline was discontinued and she was given acetazolamide 500 mg twice daily. One month later, both six nerve palsy and papilledema improved (bottom)
Investigation and Diagnosis
The diagnostic criteria of IIH was created by Dandy in 1937 and was last formulated by Friedman in 2002 [88]. The modified diagnostic criteria emphasize a normal cerebrospinal fluid composition as well as brain imaging study using MRI and MR venography (MRV) to rule out intracranial pathologies that may cause secondary intracranial hypertension. Typical symptoms of headache, pulse-synchronous tinnitus, and papilledema in overweight patients in the right demographic group make the diagnosis predictable. Diagnostic procedures include MRI and MRV of the brain and a lumbar puncture. Lumbar puncture provides information about the opening pressure as well as cerebrospinal fluid profile; the latter is essential in excluding secondary causes such as inflammation or infection. When performed without anesthesia with patients lying in the lateral decubitus position, the intracranial pressure in normal adults ranges from 100 to 250 mmH2O [88, 89]. MRI and MRV of the brain provide information to rule out intracranial pathologies such as space-occupying lesions, hydrocephalus, Chiari malformation, and cerebral venous thrombosis. The radiographic findings suggesting IIH include posterior globe flattening, optic nerve sheath distension, and an empty sella [90]. One study using a specially designed MRV protocol found stenosis in the transverse and sigmoid sinuses to be both sensitive and specific for IIH [70].
Management
The severity of the vision loss in patients with IIH is the main determinant for treatment strategy. Treatment of patients with severe headache but intact visual function and mild papilledema is more variable across practitioners. Lumbar puncture, while serving as one of the mainstays of diagnosis, sometimes results in relief of headache for a prolonged period of time [91].
Weight loss serves as an important step in the management of IIH, and successful reduction of body weight of 5–15 % may have a significant impact on the evolution of both headache and papilledema [92, 93]. Pharmacologic treatment commonly includes carbonic anhydrase inhibitor and topiramate, or methazolamide or furosemide can be used when acetazolamide is poorly tolerated [94]. One must be aware that patients diagnosed with IIH may suffer from a headache disorder above and beyond that related to the elevated intracranial pressure, given the high predilection of headache disorder in the same demographic population.
When there is imminent visual loss, surgical intervention may be required. Optic nerve sheath fenestration creates a window or multiple slits on the intraorbital segment of the optic nerve sheath behind the globe to release cerebrospinal pressure [95]. Optic nerve sheath fenestration is generally regarded as a low-risk procedure, although serious complication may rarely occur such as central retinal artery occlusion, resulting in profound loss of vision. Lumboperitoneal and ventriculoperitoneal shunts divert cerebrospinal fluid from the spinal canal or cerebral ventricle into the abdomen via a catheter to lower the intracranial pressure. This allows treatment of both the papilledema and headache and leads to stabilization or improvement of visual acuity in a suboptimal proportion of patients. However, revision surgery is frequently necessary, and other complications (as low-pressure headache, infection, arachnoiditis of nerve roots) might develop [96, 97]. Cerebral shunting procedures are generally reserved as a last measure when other treatments have failed to halt progression of vision loss, or if there is a sign of rapid, fulminant vision loss at initial presentation.
Endovascular Treatment
The established surgical methods of treatment of idiopathic intracranial hypertension have limitations. As discussed, lumbar or ventriculoperitoneal shunting typically leads to stabilization or improvement of vision acuity in a proportion of patients, but revisional surgery is frequently necessary, and other complications (aforementioned) might develop. Likewise, optic nerve fenestration has been associated with deterioration of visual function years after an initial period of stabilization. Moreover, it does not treat the underlying mechanism that leads to headaches and tinnitus [98, 99].
Venous sinus stenting is an endovascular treatment option for IIH. Although a causal link between transverse-sigmoid venous sinus stenosis and IHH has never been definitively established, this abnormality is clearly more common in individuals with IHH than controls. Patients with IHH have been described to have substantial bilateral sinovenous stenoses in 27 of 29 (93 %) patients with IIH versus 4 of 59 (6 %) in control patients by MR gadolinium-enhanced venography [70]. Furthermore, significant pressure gradients have been demonstrated by direct venous manometry in patients with IHH and transverse-sigmoid junction stenosis: venous hypertension in the superior sagittal/transverse sinuses with a significant drop in venous pressures at pre-stenotic more caudal levels (sigmoid sinus/jugular bulb) [76]. Mounting evidence indicates that stenting of the stenotic dural venous sinus maintaining the drainage system fully patent is an effective approach for selected patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








