Chapter 14 Neurologic Aspects of Chronic Pain
Pain Varieties
Unlike the dull ache of nociceptive pain, neuropathic pain consists of electric, sharp, lancinating, or burning sensations. Also, not confined to the site of tissue injury, neuropathic pain and spontaneously occurring painful paresthesias radiate throughout the distribution of the injured nerve and often well beyond it. Other features are that painful or even neutral stimuli elicit an intense, distorted, or prolonged response – allodynia, hyperalgesia, and hyperpathia (see Chapter 5).
Pain Pathways
Central Pathways
The PNS fibers enter the CNS at the spinal cord’s dorsal horn and, either immediately or after ascending a few segments, synapse in its substantia gelatinosa (Fig. 14-1). At many of these synapses, the fibers release an 11-amino-acid polypeptide, substance P, which constitutes the major neurotransmitter for pain at the spinal cord level.
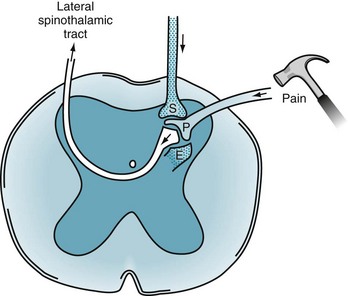
FIGURE 14-1 Painful sensations travel along the peripheral nerves’ A delta and C fibers. These fibers enter the dorsal horn of the spinal cord where, using substance P (P), they synapse on to second-order neurons. The second-order neurons cross to the contralateral side of the spinal cord and, forming the lateral spinothalamic tract, ascend to the thalamus. Two powerful pain-dampening or pain-modulating analgesic systems (stippled) play upon the dorsal horn synapse. One tract descends from the brain and releases serotonin (S). The other system is composed of spinal interneurons that release enkephalins (E).
After the synapse, pain sensation ascends predominantly within the lateral spinothalamic tract to the brain (see Figs 2-6 and 2-15). This crucial tract crosses from the substantia gelatinosa to the spinal cord’s other side and ascends, contralateral to the injury, to terminate in specific thalamic segments. Additional synapses relay the stimuli to the somatosensory cerebral cortex, enabling the individual to locate the pain.
Analgesic Pathways
Many analgesic pathways interfere with pain transmission within the brain or spinal cord. Several pathways that originate in the frontal lobe and hypothalamus terminate in the gray matter surrounding the third ventricle and aqueduct of Sylvius (periaqueductal gray matter). They contain large amounts of endogenous opioids, which are powerful analgesics (Box 14-1). Implanted electrodes that stimulate the periaqueductal gray matter area may provoke the release of endogenous opioids and thereby induce profound analgesia.
Box 14-1
Glossary
β-endorphin: An endogenous opioid concentrated in the pituitary gland and secreted with ACTH. It consists of amino acid numbers 61–91 of β-lipotropin and gives rise to the enkephalins (see Fig. 14-2)
β-lipotropin: A 91-amino-acid polypeptide, which may be an ACTH fragment. It gives rise to β-endorphin but has no opioid activity itself (that is, β-lipotropin is not an endogenous opioid)
Dynorphin: An endogenous peptide opioid that binds to kappa opioid receptors
Endogenous opioids: Polypeptides (amino acid chains) found within the central nervous system that create effects similar to those of morphine and other opioids. The effects of both endogenous and exogenous opioids are characteristically reversed by naloxone
Endorphins: Endogenous morphine-like substances or opioid peptides. This term is virtually synonymous with endogenous opioids
Enkephalins: Short (5-amino-acid) polypeptide endogenous opioids that include met-enkephalin and leu-enkephalin. They are found primarily in the amygdala, brainstem, and dorsal horn of the spinal cord
Naloxone (Narcan): A pure opioid antagonist that reverses the effects of endogenous and exogenous opioids
Substance P: An 11-amino-acid polypeptide that is probably the primary pain neurotransmitter at the first synapse of the primary afferent neuron in the spinal cord
Endogenous Opioids
Often called endorphins (endogenous morphine-like substances), endogenous opioids – endorphins, enkephalins, and dynorphins – are powerful analgesic, amino-acid chains (polypeptides) synthesized in the CNS (Fig. 14-2). Endogenous opioids bind to receptors in the limbic system, periaqueductal gray matter, dorsal horn of the spinal cord, and other CNS sites. Neurologists commonly say that a “runner’s high” and the initial painlessness reported by wounded soldiers serve as examples of endorphins’ analgesic effects.
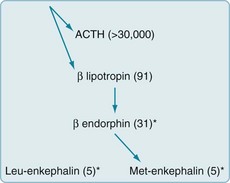
FIGURE 14-2 Endogenous opioids are synthesized and secreted, along with adrenocorticotropic hormone (ACTH), from the pituitary gland in times of stress or acute pain. A large precursor molecule (not pictured) gives rise to ACTH and β-lipotropin, which are often released together. β-lipotropin gives rise to β-endorphin and met-enkephalin, but another precursor gives rise to leu-enkephalin. (The asterisks denote the important endogenous opioids, and the numbers within parentheses are the amino acid units in the polypeptide chains.)
Treatments
Nonopioid Analgesics
As previously mentioned, aspirin, other salicylates, NSAIDs, steroids, and acetaminophen – nonopioid analgesics – inhibit prostaglandin synthesis at the injury (Box 14-2). Through this mechanism, these medicines relieve acute and chronic pain of mild to moderate severity.
Box 14-2
Examples of Analgesics
Nonopioid analgesics generally provide steady analgesia for weeks to months and avoid several potential problems. In particular, after completing a course of treatment, patients do not experience withdrawal symptoms. Also, except for high-dose steroids potentially causing steroid psychosis (see Chapter 15), these analgesics do not induce mood, cognitive, or thought disorders.
Opioids
When chronic pain results from cancer, neurologists categorize it as “cancer” or “malignant” pain, but when it results from other conditions, “noncancer” pain. Opioids are unquestionably indicated for cancer pain (see Box 14-2). In addition, a number of studies suggest that they are indicated for chronic noncancer pain syndromes.
Other Opioid Side Effects
To prevent or alleviate opioid-related nausea, physicians should prescribe antiemetics, but they should cautiously prescribe antiemetics containing phenothiazine or other dopamine-blocking agent because they can cause dystonic reactions or parkinsonism (see Chapter 18). In another caveat, pharmacologic marijuana, such as dronabinol (Marinol) and nabilone (Cesamet), generally reduces nausea, pain, and anxiety; however, robust formal studies have not established that they are the most effective treatment when cancer or chemotherapy has been responsible for these symptoms. Moreover, they may cause transient mood and thought disorders.
As with other medicines, physicians should discontinue opioids when unnecessary. Rather than abruptly stopping opioids, which would probably lead to withdrawal, physicians might use an exit strategy of tapering opioids by reducing their dose by 50% every 3 days. Alternatively, physicians might briefly replace a short-acting opioid, such as morphine, with a long-acting one, such as methadone, and then prescribe a nonopioid analgesic. If withdrawal still complicates the process, benzodiazepines may alleviate the physical or mental discomfort, and clonidine (Catapres) may blunt autonomic nervous system hyperactivity (see Chapter 21).
Chronic Opioid Therapy Debate
Physicians who have opposed expanding opioid prescription offer several counterarguments. Opioids reduce pain less than 2–3 points on a 0–10 scale. Chronic opioid therapy may be counterproductive in several conditions. It portends worse long-term outcomes for headache and low back pain, and tends to convert episodic migraines or tension-type headache to persistent daily headache (see Chapter 9). Patients demand opioids when less potent or alternative measures, even nonpharmacologic ones, would suffice. In addition, patients may subvert medical treatment. They may seek opioids for their euphoric effect rather than for pain relief. Falsely reporting the persistence and severity of pain to obtain opioids, patients prolong their disability. Some patients, claiming intractable pain, demand excessive quantities, but sell or pass along their opioids. To avoid this “diversion” of opioids, some physicians go so far as to ask the patient who has been prescribed fentanyl patches to return the used ones, which should have the patient’s hair stuck to the patch’s adhesive. Some patients use opioids to tide them over an addiction to illicit drugs. For many patients, obtaining opioids takes over their day-to-day concerns.









