Vicki L. Shanker
GASTROINTESTINAL DISTURBANCE AND NEUROLOGIC DISEASE
Gastrointestinal dysfunction can often manifest as cerebral impairment in the older adult. Changes can be as subtle as short-term memory loss or difficulty with concentration. Often, these signs are the initial or only presentation of a gastrointestinal disturbance. To achieve a rapid diagnosis, it is valuable for the primary care physician to be alert and aware of these presentations. This chapter provides a brief overview of the major gastrointestinal disturbances associated with neurologic complications in the elderly.
HEPATIC ENCEPHALOPATHY
Hepatic encephalopathy is a neuropsychiatric syndrome that may occur secondary to hepatocellular failure. The development of hepatic encephalopathy is a poor prognostic sign associated with 1-year mortality rates close to 60%. Hepatitis, cirrhosis, and portosystemic shunting are common causes. The clinical presentation of hepatic encephalopathy has been categorized in the literature into four separate stages (Table 29-5).
The first stage is notable for subtle neuropsychiatric impairment and changes in the patient that may only be recognizable by close family and friends. Symptoms include extreme mood disturbances, slurred speech, and mild confusion. A study of 148 pre-liver transplantation candidates reported significantly impaired immediate and delayed memory, attention, and executive functioning on neuropsychological testing (44). Intellectual function is initially preserved. Asterixis may be present on examination. This is a negative myoclonus where brief loss of tone can appear as hand flapping during wrist extension.
Table 29-5. Hepatic Encephalopathy: Characteristics of Encephalopathic Stages | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
As the disease process continues, intellectual and motor functions are impaired, and basic activities of daily life become challenging. Consciousness may fluctuate. Signs on examination include hypotonia, hyporeflexia, and a positive Babinski sign. Comatose states dominate end-stage hepatic encephalopathy.
The pattern of encephalopathy described is similar to that seen in other metabolic disturbances. However, some signs have been identified as more specific to liver dysfunction. These include signs of parkinsonism such as tremor, monotonous speech, bradykinesia, and diminished facial expression (hypomimia). Increased muscle tone and dyskinesias may be seen as well (31).
Encephalopathic changes emerge over a period of days to weeks. In most patients, the disease does not develop past the first two stages. However, for patients in whom it does, the course is not necessarily unidirectional. The disease course can fluctuate between levels of impairment, or it can steadily worsen. Depending on the cause, the course of the disease can be acute, subacute, or chronic. A precipitating factor is often identified in an acute presentation but occurs variably in chronic forms (Table 29-6).
Table 29-6. Common Precipitators of Hepatic Encephalopathy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
When the causative factor is identified and then eliminated or treated, resolution of the symptoms usually follows. In the absence of a precipitating event, prognosis is poor.
Diagnosis
Diagnosis is based primarily on history, physical examination, and a strong clinical suspicion. Hepatic encephalopathy is a diagnosis of exclusion, and potential confounders must be ruled out prior to its diagnosis. Predisposing factors of encephalopathy include an underlying infection, dehydration, electrolyte dysfunction, hypotension, gastrointestinal bleeding, and hepatocellular cancer. Additionally, excessive use of medications such as benzodiazepines, psychoactive drugs, or alcohol may produce an encephalopathy (16).
The workup focuses on evidence of hepatocellular insufficiency and mental impairment. Test results are consistent with, but not specific to, hepatic encephalopathy. Laboratory tests such as elevated liver function tests, increased prothrombin time, and raised ammonia levels may indicate hepatic dysfunction. Of note, ammonia levels are not always elevated with hepatic dysfunction. When ammonia levels are elevated, they do not consistently correlate with the degree of hepatic encephalopathy. Brain imaging is also nonspecific. Computed tomography (CT) is useful in these cases to rule out other considerations in the differential diagnosis. The main magnetic resonance imaging (MRI) findings reported in hepatic disease are symmetric pallidal hyperintensities in T1-weighted images. The nigral substance area and the dentate cerebellar nucleus may show similar abnormalities. Electroencephalograph (EEG) findings of triphasic waves are commonly found but are nonspecific. A referral to a neuropsychologist is valuable in further assessment of mental dysfunction in hepatic encephalopathy.
Treatment
Treatment focuses largely on reduction of systemic ammonia and on inhibition of gamma-aminobutyric acid (GABA) receptor activation. Both have been identified as contributors to the pathophysiology of hepatic encephalopathy. Elevated ammonia levels augment GABA-mediated neurotransmission, increasing neuronal inhibition.
Nonabsorbable disaccharides (e.g., lactulose and lactitol) are used to osmotically remove ammonia from the body. They have traditionally been used as a first-line therapy based on the theory that the majority of ammonia production stems from colonic bacteria. The estimated daily dose ranges from 30 to 60 g. The dose is titrated to produce two to four soft acidic (pH <6) stools per day. In an acute episode, the recommended starting dose is 30 to 45 mL at 1- to 2-hour intervals until a laxative effect is observed. Elderly patients taking lactulose should be monitored closely for fluid and electrolyte loss with chronic use because they are more susceptible to neurologic impairment associated with dehydration and electrolyte loss than are younger patients.
Antibiotics have been used as a second-line approach. Neomycin (6 g/day) and metronidazole (800 mg/day), inhibitors of urease-producing bacteria, are used to reduce ammonia. In the geriatric population, serum concentrations of these medications tend to be elevated as total clearance is reduced. Thus, elderly patients are more likely to suffer from side effects, including ototoxicity, nephrotoxicity, and gastrointestinal disturbance. Rifaximin (1,200 mg/day) is a nonabsorbable alternative to neomycin and metronidazole. A small pilot study of patients with stage 2 hepatic encephalopathy compared the cost efficacy (via number of hospitalizations, medical treatments, and drug costs) in a group taking rifaximin versus a group taking lactulose (48). Researchers found that patients taking rifaximin required fewer hospitalizations and outpatient medical visits. They reported a significant cost efficacy of rifaximin over lactulose, begging further study of this drug. The question of an additive effect of lactulose with antibiotics is currently under investigation as well. Of note, studies of patients receiving antibiotics for liver disease have found that stool pH is increased, a sign that disaccharidemetabolizing bacteria are killed by the antibiotics as well (66). Absence of these bacteria reduces the effectiveness of lactulose.
Dietary protein restriction is another first-line approach to reducing intestinal production of ammonia. Patients are restricted to 20 g/day of protein. The
dosage is increased by 10 g for 3 to 5 days until a tolerance threshold is reached. If tolerance is <1 g/kg, vegetable protein is recommended as the primary source of protein because of its high fiber content. Increased fiber intake aids in improved food motility through the gastrointestinal system, decreasing the opportunity for protein absorption.
dosage is increased by 10 g for 3 to 5 days until a tolerance threshold is reached. If tolerance is <1 g/kg, vegetable protein is recommended as the primary source of protein because of its high fiber content. Increased fiber intake aids in improved food motility through the gastrointestinal system, decreasing the opportunity for protein absorption.
Recently, researchers have begun to question the basis of evidence for the common usage of lactulose and protein restriction in the treatment of hepatic encephalopathy. Shawcross and Jalan (57) reviewed 22 randomized trials of lactulose and found insufficient evidence to recommend or refute its use. Use of lactulose was not found to significantly reduce mortality. With regard to dietary practices, a small trial of 20 patients with hepatic encephalopathy placed half of the participants on a normal protein diet and half on a restricted diet (14). There were no significant differences in disease course or in protein breakdown between these two groups. Studies such as these have suggested that research and, ultimately, clinical treatment may be redirected to a more systemic approach of ammonia reduction and elimination than currently understood and practiced.
Other current strategies for treatment of hepatic encephalopathy include the eradication of Helicobacter pylori, if present, because of its contribution to serum ammonia levels. Ornithine aspartate, an enzyme that converts ammonia to urea, is effective in serum ammonia reduction and can be taken orally. A recent trial found significant improvement in serum ammonia levels, EEG recordings, and mental status testing in a group taking oral ornithine aspartate (51). Benzoate or phenylacetate treatment reacts with glycine or glutamine, respectively, to increase metabolic conversion to form hippurate and phenacetylglutamine. Zinc is a necessary cofactor in two of the five enzymes in the urea cycle. Supplementation is recommended for those who are zinc deficient. Studies have explored the role of flumazenil, a benzodiazepine antagonist, in hepatic encephalopathy. Some have proposed that binding to the benzodiazepine receptor by substances not normally present in the brain plays an important role in hepatic encephalopathy. A Cochrane review (2) assessed the results of 13 randomized trials using flumazenil in the treatment of hepatic encephalopathy. It concluded that flumazenil had significant benefit in short-term improvement in patients with cirrhosis.
Liver transplantation is preferable to medical management in most patients with end-stage cirrhosis, regardless of age. However, other medical problems may limit the utility of this option. The surgical creation of portosystemic shunts, especially the transjugular intrahepatic portosystemic shunt, is a frequently used procedure in those awaiting transplantation. However, patients >65 years of age are significantly more likely to develop hepatic encephalopathy after this procedure.
NUTRITIONAL AND VITAMIN DEFICIENCY
As the body ages, the efficiency of the gastrointestinal system to extract the necessary nutrients and vitamins from the diet can become impaired. External factors can further compound the risk of nutritional deficiency. For example, patients’ medications may interfere with absorption, and patients’ diets, especially individuals who are institutionalized, may be inadequate (12).
Vitamin B12 deficiency is a common problem in the elderly community. There are variable estimates of its prevalence, ranging from 12% of those living in the community to 30% to 40% of those in institutions (38,62). Some of this discrepancy is due to the variable criteria used to define vitamin B12 deficiency, with the lower limit of normal ranging from 150 pmol/L to 258 pmol/L. The peak age of neurologic presentations in vitamin B12 deficiency is estimated to occur between 60 and 70 years (52).
Gastric atrophy is among the most common changes in the aging gastrointestinal system. Estimates of atrophic gastritis in the geriatric population range from 11% to 50% (28,55). States of hypochlohydria or achlorhydria facilitate the atrophy of the stomach mucosa. H. pylori infection in the gut is the most frequent cause of atrophic gastritis, with an estimated 60% of the elderly infected. Pernicious anemia, another common source, is estimated to produce 15% to 50% of vitamin B12 deficiency cases in the elderly (3). Classic pernicious anemia is a chronic illness associated with lack of adequate intrinsic factor production secondary to inhibition of gastric parietal cells. Intrinsic factor is needed for the absorption of vitamin B12.
There are several other etiologies that may contribute to vitamin B12 malabsorption. Bacterial overgrowth from lack of gastric acid production or chronic antibiotic use also produces nutritional malabsorption. Long-term ingestion of medications such as metformin, H2 receptor antagonists, proton pump inhibitors, anticonvulsants, and colchicine may cause malabsorption. Other etiologies include a history of gastric surgery, chronic alcoholism, pancreatic exocrine failure, and Sjögren syndrome. Vitamin B6, vitamin B12, and folate are most notably affected by these changes.
The neurologic manifestations of vitamin B12 deficiency are well known and reported. Deficiency states may involve both the central and peripheral nervous system. Onset is often insidious. Nerve damage is usually due to demyelination. However, axonal
degeneration and neuronal death can occur in more severe cases. Initial symptoms may be as subtle as lethargy or generalized weakness. The most common symptomatic presentation is burning or painful sensations in the distal extremities. The feet are usually affected prior to the hands. These symptoms are associated with polyneuritis. Cognitive symptoms may occur with or without peripheral involvement. Symptoms may range from mild irritability to depression, dementia, and psychosis. On rare occasions, there is optic nerve involvement, and patients may complain of visual loss.
degeneration and neuronal death can occur in more severe cases. Initial symptoms may be as subtle as lethargy or generalized weakness. The most common symptomatic presentation is burning or painful sensations in the distal extremities. The feet are usually affected prior to the hands. These symptoms are associated with polyneuritis. Cognitive symptoms may occur with or without peripheral involvement. Symptoms may range from mild irritability to depression, dementia, and psychosis. On rare occasions, there is optic nerve involvement, and patients may complain of visual loss.
The most frequent neurologic signs on examination are a diminished sense of vibration and proprioception in the legs due to involvement of the dorsal columns of the spinal cord. This may occur in conjunction with impaired distal cutaneous sensation. Sensory changes are usually in a stocking-glove distribution. Limb reflexes may vary. Subacute combined deficiency, a condition classically associated with vitamin B12 deficiency, involves both posterior columns and corticospinal tracts of the spinal cord. A spastic paraparesis is seen on examination. Involvement of the autonomic nervous system (ANS), including bowel, bladder, and sexual dysfunction, can be seen in some cases as well.
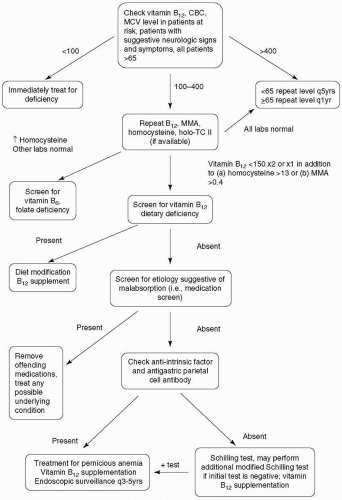
A suggested algorithm for evaluation of vitamin B12 deficiency is presented in Figure 29-1. When patients present with symptoms suggestive of vitamin B12 deficiency, a serum screen of vitamin B12 should be sent along with a complete blood cell count (CBC). Vitamin B12 levels <100 pmol/L warrant immediate therapy. When levels between 100 and 400 pmol/L are reported, additional serum tests should be sent (19). These include a homocysteine, methylmalonic acid, and holotranscobalamin (holo-TC) II assay. The former two are both in the pathway of vitamin B12 production, and elevated levels are suggestive of deficiency. Elevated homocysteine is a nonspecific finding because it is also involved in the pathway of folate production. Elevated methylmalonic acid is a more specific finding. Holo-TC contains biologically active vitamin B12 bound to transcobalamin II (TC II). TC II promotes vitamin B12 uptake by cells. Low serum holo-TC is considered the earliest and most sensitive marker for vitamin B12 deficiency (68). If additional tests confirm the presence of vitamin B12 deficiency, serologies for pernicious anemia should be performed. This includes an anti-intrinsic factor antibody, which has 50% sensitivity and 98% specificity, and an antigastric parietal cell antibody, which has 90% sensitivity and 50% specificity.
The Schilling test can be of assistance in identifying the source of vitamin B12 deficiency. The test is often separated into three separate stages. In the first phase of the test, the patient is given an oral dose of radiolabeled vitamin B12 followed by an intramuscular injection 2 hours later. The purpose of this is to saturate the body’s vitamin B12 binding sites. The absorbed oral vitamin B12 cannot bind to the already saturated transcobalamin proteins and will be excreted in the urine by glomerular filtration. A percentage of the administered dose excreted in the urine over 24 hours is calculated. In patients with pernicious anemia or intestinal malabsorption, the urinary excretion is usually <6%, compared with the normal value, which is >9%. The second phase is performed in a manner similar to the first phase, with the exception that intrinsic factor is given with the oral dose of vitamin B12. Pernicious anemia is suggested when the patient has an abnormal result in phase I followed by a normal test in phase II. When the Schilling test suggests pernicious anemia and anti-intrinsic factors are present, the diagnosis of pernicious anemia is virtually confirmed (specificity >99%). If the first two tests produce abnormal results, the patient is treated with 2 weeks of tetracycline for possible bacterial overgrowth. Phase III of the Schilling test is performed after a 2-week course of antibiotic. The test is the same as the second phase. If the test is normal, it can be deduced that bacterial overgrowth caused the vitamin deficiency. Of note, the test results can be normal in elderly patients with atrophic gastritis, despite deficiency, because these patients malabsorb only protein-bound vitamin B12.
When vitamin B12 deficiency is suspected, the clinician should obtain a good history, including diet and medications. There are several options for supplementation of vitamin B12 once deficiency is identified. Traditionally, patients were given a 1,000 µg/day intramuscular (IM) injection for 1 week followed by a monthly 1,000 µg/day IM injection until the deficiency is corrected or for life. Recently, studies have argued that initial oral supplementation of 1,000 to 2,000 µg daily, followed by weekly and then monthly doses, is equally efficacious (37,63). Patients with confirmed pernicious anemia should be referred to a gastroenterologist. These patients should receive endoscopic surveillance every 3 to 5 years with multiple biopsies because of the association of pernicious anemia with gastric cancers including lymphoma, adenocarcinoma, and carcinoid tumors. Screens for other autoimmune conditions can be considered if symptomatically appropriate. Antibiotic treatment for H. pylori should be administered, if detected. The length and extent of neurologic recovery is correlated with the length and severity of disability prior to treatment. Older age may increase the risk of residual disability. Patients with suspected vitamin B12 deficiency should be cautioned against receiving nitric oxide during dental procedures because exposure may precipitate or rapidly advance current symptoms.
Folate is found in leafy green vegetables and is supplemented in many foods. Despite this supplementation, evidence suggests that 30% of healthy older adults may continue to have deficiencies (15). Folate deficiency may be subtle and is not fully understood. The relationship of low folate states with depressive mood disorders is still under study. It is reported that patients with low levels of serum folate have poorer responses to antidepressant medication (29). When levels are low, folate supplementation is provided. There is no standard for the recommended dose used for supplementation. However, 0.8 mg/day taken over several months is usually sufficient to correct anemia (52). Treatment should continue for at least 6 months. Some treatment response should be seen by 3 months.
Precaution must be taken when using the mean corpuscular volume (MCV) value alone for screening or measuring treatment effects in patients with cobalamin or folate deficiency. Lack of adequate amounts of either vitamin impairs DNA synthesis, resulting in a megaloblastic anemia. A well-supplemented diet with folate may delay the identification of a vitamin B12 deficiency. Chronic treatment with folate or vitamin B12 supplementation may mask an emerging deficiency of the alternative vitamin, allowing neurologic damage to advance. This is why both cobalamin and folic acid should be assessed when symptoms are suggestive, regardless of CBC results.
Pyridoxine deficiency is reported to be present in as many as 32% of independent American elderly (40). Risk factors include older age, renal insufficiency, dialysis treatment, inflammatory disease, and chronic malnutrition. Certain medications may contribute to or provoke a pyridoxine deficiency. These include hydralazine, isoniazid, levodopa, D-penicillamine, and pyrazinamide. Unlike children, adults are better able to withstand low vitamin B6 states and will often not display any clinical manifestations of insufficiency. Seizures in adults are rare. More common are slowly progressive sensory changes resulting in numbness, tingling, burning, or pain in the feet that may travel in a stocking-glove pattern to affect the hands as well, if left untreated. Examination may show impaired distal sensation, distal weakness, and decreased reflexes. Pyridoxine supplementation of 50 mg/day is sufficient for prevention and treatment of deficiency.
Folate, vitamin B6, and vitamin B12 are all cofactors involved in homocysteine metabolism (59). Elevated levels of homocysteine are associated with increased risk of atherosclerotic disease and stroke (26,41). Hyperhomocysteinemia has been associated with late-onset mood disorder in the elderly as well (13). When elevated states of serum homocysteine are detected, it is important to screen and supplement the appropriate vitamins. Several studies have suggested that there is a relationship between elevated homocysteine levels and cognitive decline (10,21,46). Unfortunately, the current literature does not suggest that lowering homocysteine levels via B vitamin supplementation improves cognitive function (43).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree