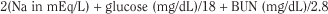CHAPTER 116 NEUROLOGY OF COMMON ELECTROLYTE DISORDERS
HYPEROSMOLALITY AND HYPERTONICITY
Normal serum, and therefore body fluid, osmolality is in the range of 275 to 295 mOsm/kg; clinically significant effects are generally seen at levels greater than 325 mOsm/kg. Osmolality may be measured directly by the freezing point depression or calculated as serum osmolarity in milliosmoles per liter with the following formula, which accounts for the millimolar quantities of major serum solutes (where BUN is blood urea nitrogen):
Azotemia is caused by renal failure or inadequate renal perfusion (prerenal azotemia).
The treatment of hyperosmolality requires calculation of apparent water losses:
2. The total body solute (sodium + potassium) is estimated as NTBW × 140 mEq/L. Note that 140 mEq/L is normal serum [Na] but is approximately equal to intracellular [K] needed to estimate total body solutes.
5. Apart from deficit correction, large ongoing losses in the urine (osmotic diuresis or diabetes insipidus) or sweat (fever) are estimated and replaced.
Only gold members can continue reading. Log In or Register to continue