Congenital
1. With regards to brain development, what are the primary brain vesicles? What secondary brain vesicles do they give rise to?
The neural tube gives rise to the following primary followed by secondary brain vesicles (Fig. 3.1)1:
— Forebrain (prosencephalon)
• Telencephalon
• Diencephalon
— Midbrain (mesencephalon)
• Mesencephalon
— Hindbrain (rhombencephalon)
• Metencephalon
• Myelencephalon
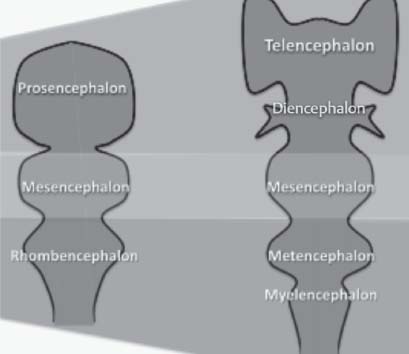
Fig. 3.1 Neural tube development: Primary and secondary brain vesicles.
2. Compare primary and secondary neurulation.
A. Primary neurulation: Describes the early embryologic formation of the neural plate and its subsequent closure forming the neural tube. It includes
• Formation of the neural plate (by the neurectoderm)—day 18 and 19
• Development of the neural folds—day 20 to 21
• Closure of the neural tube—starts at day 22, then the rostral neuropore closes at day 24 and the caudal neuropore at day 26.
• The resulting neural tube will eventually develop into the brain and spinal cord down to the second sacral level.
B. Secondary neurulation: Describes the development of the neural tube (day 28). It is species specific; in chicks, the tube forms by canalization and multiple luminal secondary neural tubes coalesce and become the primary neurulation tube at the overlap zone.
• In mice, there is extension of the primary neural tube into the medullary rosette that is formed by recruiting cells from the caudal cell mass (CCM) beyond the second sacral level (S2).1,2
3. At what spinal level does the spinal cord end, during pre- and postnatal life?
— Prenatal life:
• 12 weeks at C5
• 15 weeks at S3
• 24 weeks at S1
— Postnatal life:
• Newborn at L3
• Adult at L1–L2, end of dural and arachnoid sac at S21
4. Classify dysraphism according to embryology.
— Disorders during gastrulation (week 3, trilaminar):
• Split cord malformation
• Combined spina bifida
• Neurenteric cysts
• Some myelomeningoceles
• Hemimyelomeningoceles
• Klippel-Feil syndrome
• Complex dysraphic malformations
— Premature ectodermal dysjunction:
• Lipomyelomeningocele
— Disorders during primary neurulation (week 4):
• Anencephaly
• Cranioschisis
• Myelomeningocele
• Myeloschisis
— Disorders during secondary neurulation (week 4):
• Dermal sinus tract
• Dermoid or epidermoid tumors
• Spina bifida occulta
— Postneurulation disorders:
• Encephalocele
• Meningocele
• Chiari II malformation2
5. How does tissue repair differ in the fetus brain compared with the adult brain?
In early fetal life, up to the first half of the second trimester, if ischemia occurs, macrophages are attracted to the area, phagocytosis takes place (without gliosis), producing a smooth-walled defect, which would give a pseudoprimary malformation. This is the case with hydranencephaly or a porencephalic cyst that occurs from an insult before or at early second trimester.2
6. In which patients do subependymal germinal matrix (GM) hemorrhages occur?
GM hemorrhage occurs in prematurely born (usually before 34 weeks of gestation) and low-birth-weight neonates (50% in neonates < 1500 kg) mostly, within the first 3 days after delivery.3,4
7. What is the pathogenesis of GM hemorrhage?
— GM has the following characteristics:
• Located in the periventricular area
• Contains fragile microcirculation stroma
• Has high levels of tissue plasminogen activator that may impair hemostasis, and receives a major percentage of the cerebral blood flow
— The GM starts involution at 34 weeks of gestation.
— In case of hypoxic stress, autoregulation fails and excessive perfusion ruptures the GM microcirculation leading to hemorrhage.3,4
8. What are the grades of GM hemorrhage?
I. Confined only to GM
II. Hemorrhage filling lateral ventricles without distension
III. Hemorrhage filling and distending lateral ventricles
IV. Hemorrhage extending into the neighboring parenchyma (Fig. 3.2)5
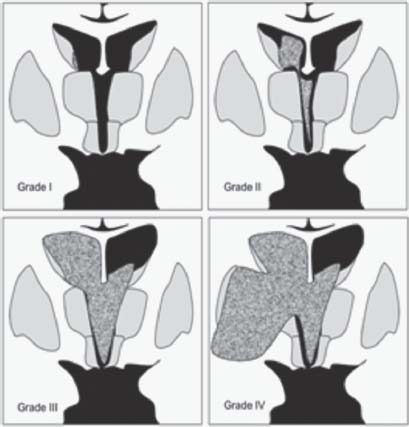
Fig. 3.2 The four grades of germinal matrix hemorrhage.
9. Where is the usual location of GM hemorrhage?
Around the GM over the head of the caudate followed by the thalamus or behind the foramen of Monro (unlike the most common site of term-baby intraventricular hemorrhage—from choroid plexus)3,4,5
Trauma
10. Where do contusions occur most frequently?
Frontal and temporal lobes6
11. What are the sources of bleeding in traumatic epidural hematoma?
The most common source of bleeding is arterial blood, mainly from the middle meningeal artery, and less commonly from a laceration of a venous sinus, such as the transverse sinus after sustaining an occipital fracture.6
12. What is a Duret hemorrhage?
A Duret hemorrhage is a delayed upper brainstem hemorrhage (anterior and paramedian aspects) that typically occurs in patients with rapidly evolving descending transtentorial herniation.7
13. What is the pathophysiology of a Duret hemorrhage?
The origin remains controversial. One theory states that it is of arterial origin, occurring due to stretching and laceration of the pontine perforating branches of the basilar artery. Another theory states a venous origin due to thrombosis and venous infarction. A multifactorial origin is more likely.7
14. What is a diffuse axonal injury (DAI)?
A DAI is the most severe form of traumatic axonal injury. It encompasses axonal damage throughout the hemispheric white matter and the rostral brainstem (95%). The regions involved are by definition the following: the parasagittal white matter, large fiber bundles such as the corpus callosum (92%), the internal capsule, the cerebellar peduncles, and possibly other sites where they are also known as gliding (88%), thalamic (56%), and caudate (17%) contusions.6
15. What is the difference between a Duret hemorrhage and brainstem small tissue-tear hemorrhages that accompany a DAI?
Both are located in the upper brainstem, but a Duret hemorrhage tends to be delayed and located in the paramedian areas, whereas DAI is typically located in the dorsolateral brainstem (Fig. 3.3).6,7
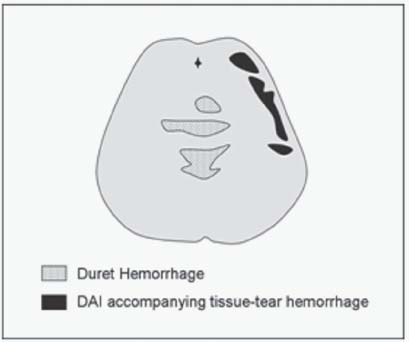
Fig. 3.3 Duret hemorrhages versus diffuse axonal injury (DAI) in the brainstem.
16. What is the microscopic timeline picture of a DAI?
Two to 12 hours. Axonal swellings (bulbs) develop, which can be verified by β-amyloid precursor protein (βAPP). Axonal disconnection follows; microglial clusters around degenerating axons appear at 5 to 10 days.6
17. Classify diffuse axonal injury (DAI).
• Stage I: DAI evident in frontal and temporal lobes mostly; also seen in cerebellar cortex and the internal capsule
• Stage II: DAI also seen in corpus callosum, especially the splenium
• Stage III: DAI also evident in brainstem and cerebellar peduncles and corticospinal tracts
18. What are the biochemical changes in DAI?8
• 1 hour: Neurofilament immunoreactivity
• 4–5 hours: Accumulation of amyloid precursor protein
• 6 hours: Ubiquitin (a marker of neural damage) is evident
• 12–24 hours: Axonal varicosities
• 1 day–2 months: Axonal swelling
• 2 months–years: Wallerian degeneration and demyelination
19. Which type of temporal bone fracture results in a conductive hearing deficit more frequently?
Longitudinal temporal bone fractures more often result in conductive hearing deficits; transverse temporal bone fractures result in disruption of the otic capsule and more often direct nerve damage with sensorineural hearing deficit. Transverse fractures are often associated with more forceful impact than longitudinal fractures; therefore, they have other associated injuries (epidural hematoma, diffuse axonal injury).9
Epilepsy
20. What are the layers of the neocortex, and what are the main cells that reside in these layers? What are their connections?
There are six layers in the neocortex. These are listed in Table 3.1 with their corresponding cell types and connections.
Layer | Cell Type by Layer | Connections |
I Molecular | Horizontal cells (of Cajal) & Golgi cells | I & II Diffuse afferent fibers from lower brain |
II External granular | Granule cells | II & III Ipsilateral corticocortico association fibers |
III External pyramidal | Pyramidal neurons | Commissural fibers |
IV Internal granular | Stellate cells | Main sensory afferents |
V Internal pyramidal | Pyramidal neurons | Main efferent supply to brain-stem/spinal cord |
VI Multiform | Fusiform or spindle cells | Efferent supply to thalamus |
21. What is the most striking microscopic finding in lissencephaly type I (agyria or pachygyria)?
In lissencephaly type I, the cortex is made up of four layers9:
1. Molecular layer
2. External neuronal layer—thin
3. Sparsely cellular layer
4. Internal neuronal layer—thick
22. Describe the connections of the mesial temporal lobe.
These connections are illustrated in Fig. 3.4 and Fig. 3.5.
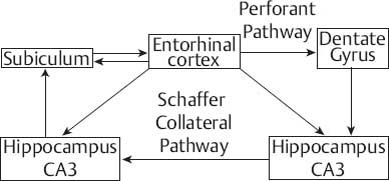
Fig. 3.4 Summary of connections of the mesial temporal lobe.
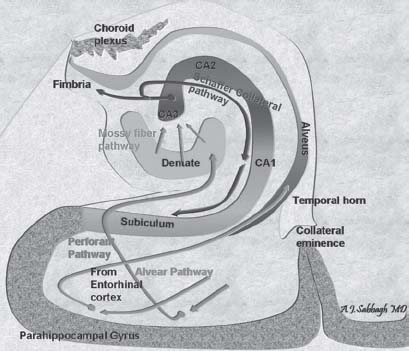
Fig. 3.5 Anatomic illustrations of the connections of the mesial temporal lobe.
23. Describe the macroscopic and microscopic picture of mesial temporal lobe (hippocampal) sclerosis.
Mesial temporal sclerosis is also known as Ammon’s horn sclerosis. Macroscopically, the involved hippocampus is smaller than the normal side and the temporal horn may be enlarged on the involved side. Microscopically, neuronal loss mainly occurs in the CA1 region, more so than the CA4, CA3, and CA2 regions and the dentate gyrus. This may be associated with some degree of gliosis (Fig. 3.6 and Fig. 3.7).10
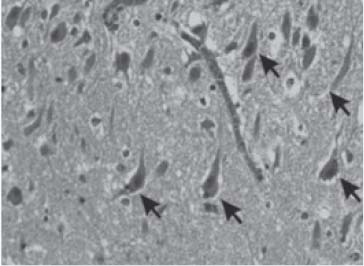
Fig. 3.6 Hippocampal sclerosis. Arrows show neurons; there is neuronal loss on the right-hand side of the image at the level of CA1. (Courtesy of Dr. Manuel Graeber—reproduced with permission)

Fig. 3.7 Reactive astrocytes. (Courtesy of Dr. Manuel Graeber—reproduced with permission)
24. What is Rasmussen syndrome?
It is a rare neurologic disorder that is usually seen in children. It is characterized by a progressive unilateral neurologic deficit, which is associated with sudden onset of epilepsy that is refractory to medical treatment. Patients usually have hemiplegia, hemianopsia, and intellectual deterioration.10
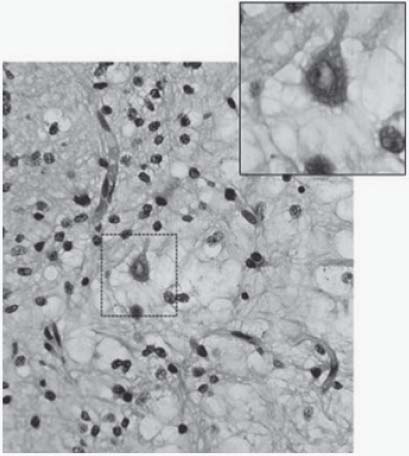
25. What is the microscopic picture of Rasmussen syndrome?
Rasmussen syndrome may have reminiscent features of chronic viral encephalitis. It is characterized by the presence of1 lymphocyte cuffs around blood vessels,2 widespread or clustered lymphocytes and microglia (or so called microglial nodules), and neurophagia.3 These features are most often unilateral and located in the neocortex, hippocampus, subjacent white matter, and basal ganglia. This is seen in addition to chronic leptomeningitis.9
26. A 19-year-old patient had a lesion removed from the right temporal lobe after having suffered from intractable epilepsy for 15 years. What is the most likely diagnosis based on the pathology slide shown in Fig. 3.8? Describe the typical finding seen in the inset box.
Dysembryoplastic neuroepithelial tumor (DNET). The finding in the inset consists of a floating neuron. DNETs microscopically are formed of oligodendrocyte-like cells in a mucinous matrix. Microcysts are found within this matrix and suspended “floating neurons” are seen in a mucopolysaccharide-rich medium.9