in the nigrostriatal system, just as much a person with Huntington’s chorea would have a normal striatum, or a patient with Pick’s or Alzheimer’s disease would have no changes in the cerebral cortex.
Table 2.3.5.1 Gross morphometric findings in schizophrenia | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 2.3.5.2 Neuronal morphometric findings in schizophrenia | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
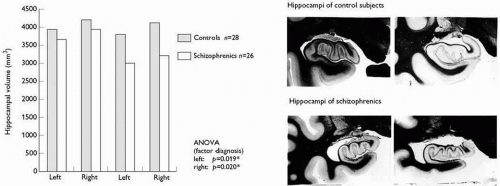
(lower row of the photographs). Other findings in limbic brain regions are left temporal horn enlargement, white-matter reductions in parahippocampal gyrus or hippocampus, and an increased incidence of a cavum septi pellucidi.
Therefore, it seems unlikely that the majority of schizophrenic patients show a considerable degree of astrogliosis. There is, on the contrary, some evidence demonstrating reduced macroglial densities in major depression and schizophrenia.(29) In this respect, specifically the oligodendroglia demonstrates qualitative and quantitative changes in schizophrenia(30) which is an interesting view of the riskgene Neuregulin-1 regulating myelin thickness via these cells. Some recent studies found evidence for the activation of microglia in the cortex of patients with schizophrenia.(31, 32, 33) As microglia respond to neuronal injury within 24 to 48 hours, studies are needed to link psychopathology with these markers. The examination of apoptic processes constitutes another interesting line of research supporting atypical degeneration with schizophrenia. Some recent studies demonstrate low-grade apoptotic processes in circumscribed brain regions in schizophrenia(34, 35) which is in line with other degenerative disorders showing similar features.(36)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree