and Héctor H. García3, 4
(1)
School of Medicine, Universidad Espíritu Santo, Santo, Ecuador
(2)
Department of Neurological Sciences, Hospital-Clinica Kennedy, Guayaquil, Ecuador
(3)
Cysticercosis Unit, Instituto Nacional de Ciencias Neurológicas, Lima, Peru
(4)
School of Sciences, Universidad Peruana Cayetano Heredia, Lima, Peru
Abstract
Almost every organ of the human economy may be affected by cysticerci, but with few exceptions, significant disease is mostly observed in patients with neurocysticercosis. The latter is a highly pleomorphic disease, defined as the infection of the central nervous system and its coverings by the larval stage of the tapeworm T. solium. The clinical pleomorphism of neurocysticercosis is mainly related to both the myriad of pathological changes that parasites may cause within the nervous system and the individual differences that exist in the severity of the host’s immune response against cysticerci which, in turn, causes a great variety of lesions in the adjacent cerebral tissue (Fleury et al. 2010; Mahanty and García 2010).
Almost every organ of the human economy may be affected by cysticerci, but with few exceptions, significant disease is mostly observed in patients with neurocysticercosis. The latter is a highly pleomorphic disease, defined as the infection of the central nervous system and its coverings by the larval stage of the tapeworm T. solium. The clinical pleomorphism of neurocysticercosis is mainly related to both the myriad of pathological changes that parasites may cause within the nervous system and the individual differences that exist in the severity of the host’s immune response against cysticerci which, in turn, causes a great variety of lesions in the adjacent cerebral tissue (Fleury et al. 2010; Mahanty and García 2010).
3.1 Microscopic Identification of the Parasite
As previously described, cysticercus is a vesicle consisting of two main parts, the vesicular wall and the scolex. When the whole vesicle is available for pathological examination, it is useful to open the vesicle until the larva is exposed. Then, the larva is placed between two glass slides which are pressed firmly until the scolex flattens; subsequent microscopic examination usually reveals the characteristic double crown of hooks and the four suckers of Taenia solium (Fig. 3.1). Examination of serial sections of the vesicle also allows the proper differentiation of Taenia solium cysticercus from the larvae of other cestodes (Tay-Zavala 1983). This permits the visualization of the spiral canal and the scolex, the latter appearing as a compact structure having in its interior several anhistous, cornified, and semitransparent structures corresponding to the hooks (Fig. 3.2).
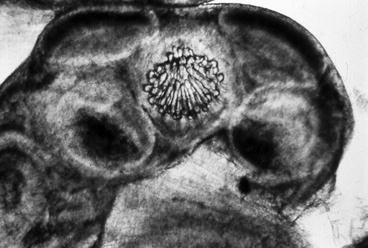
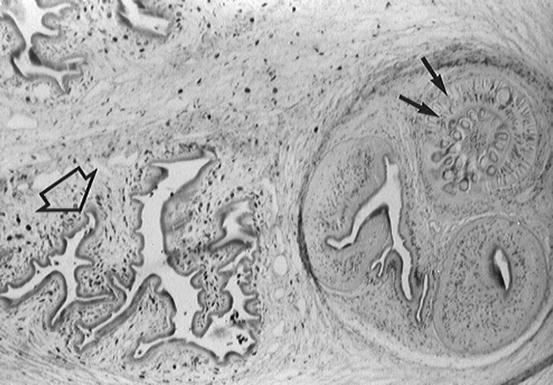

Fig. 3.1
Rostellum of Taenia solium, fresh specimen seen under light microscopy (From Del Brutto et al. (1998), with permission)

Fig. 3.2
Section of Taenia solium cysticercus showing spiral canal (open arrow) and the rostellum with characteristic hooks (small arrows) (From Del Brutto et al. (1998), with permission)
In some cysticerci—particularly those located in the subarachnoid space—the scolex cannot be identified. Instead, these parasites are mainly composed of several membranes attached to each other (Bickerstaff et al. 1952; Escobar 1983). It has been shown that membranes begin to proliferate after the scolex degenerates, showing changes in their histochemical composition, including the presence of large amounts of hydrophilic lipids (Valkounova et al. 1992).
It is common to call Cysticercus cellulosae to those parasites having a scolex and Cysticercus racemosus to those lacking the scolex. This inadequate terminology has created confusion and it has even been considered that these are unrelated parasites originating from different Taenia spp. (Biagi et al. 1961). The term Cysticercus cellulosae was coined when it was unknown that cysticerci are the larval stage of Taenia solium, and the term Cysticercus racemosus was created to describe the appearance of some cysticerci located in the subarachnoid space that tend to group in clusters resembling a bunch of grapes. Therefore, they should not be written as scientific names (in italics) and is preferable to describe these organisms as “the cellulose form” and “the racemose form” of cysticerci (Flisser 1994). Rabiela and coworkers (1985, 1989) described in detail the morphologic characteristics of the cellulose and the racemose forms of cysticerci, providing evidence that they belong to the same Taenia spp. The authors also found another form of cysticercus that conserves the scolex but has two or more small bladders sprouting from the main vesicle (Fig. 3.3). This intermediate form represents the initial stages in the transformation of the cellulose form into the racemose form of cysticercus, in which the vesicle begins to grow and the scolex disappears as the result of a degenerative process—called hydropic degeneration—that is related to the entrance of cerebrospinal fluid into the vesicles (Escobar and Weidenheim 2002).
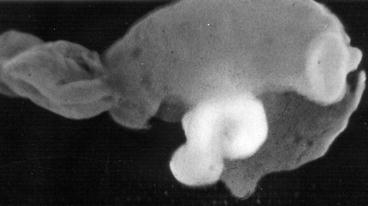

Fig. 3.3
Intermediate form of cysticercus showing typical membranes of the racemose form of cysticercus with preserved scolex (From Del Brutto et al. (1998), with permission)
3.2 Macroscopic Appearance of Cysticerci
The main factor determining the macroscopic appearance of cysticerci is their location in the central nervous system (Sotelo et al. 1996). Indeed, the size and shape of parasites vary according to whether they are situated in the brain parenchyma, the subarachnoid space, the ventricular system, or the spinal cord. Parenchymal brain cysticerci most often lodge in the cerebral cortex or the basal ganglia due to the high vascular supply of these areas, although some cysts may be seen in the brainstem or the cerebellum (Fig. 3.4). Most of these lesions measure from 5 to 15 mm in diameter, as the pressure of the brain parenchyma reduces the chance of further grow of the cysts. Sometimes, particularly in patients with the so-called heavy non-encephalitic form of neurocysticercosis (García and Del Brutto 1999), hundreds of cysts may be seen disseminated within the brain parenchyma, giving the brain a “Swiss-cheese” appearance (Fig. 3.5).
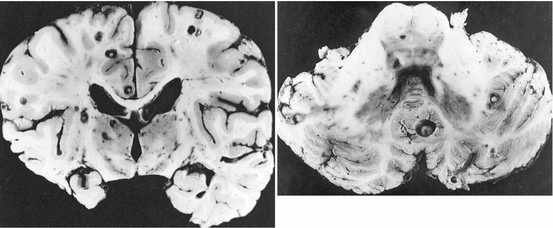
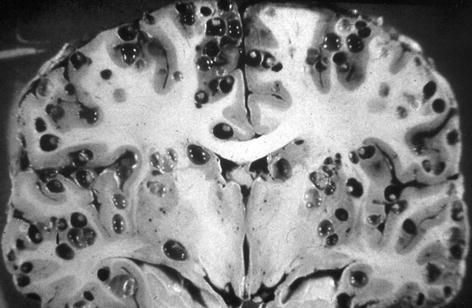

Fig. 3.4
Parenchymal brain cysticercus. Note the presence of lesions in the cerebral cortex and the basal ganglia (left). Some cysts are also located in the brainstem and cerebellum (right) (From Palmer and Reeder (2001), with permission)

Fig. 3.5
Massive non-encephalitic parenchymal brain cysticercosis. Hundreds of living cysts (showing scolices) are predominantly located in the cerebral cortex and deep within cortical sulci (From García and Martinez (1999), with permission)
Subarachnoid cysticerci located at the cortical surface of the brain or within cortical sulci between two cerebral convolutions most often have a similar shape and size than parenchymal brain cysts (Fig. 3.6). According to most neuropathologists, this is the most common location of intracranial cysticerci, as many of the cysts that appear as parenchymal on neuroimaging studies are most likely subarachnoid and located deep within cortical sulci (De Souza Lino-Junior at al. 2007; Escobar et al. 1985; Kimura-Hayama et al. 2010; Pittella 1997; Rabiela-Cervantes et al. 1982). On the other hand, cysticerci located at the Sylvian fissure or within the cerebrospinal fluid cisterns at the base of the brain (including the cerebellopontine angle cistern, the ambiens and prepontine cisterns, and the optochiasmatic region) may attain a size of 50 mm or more, as their growth is not limited by the pressure effects of the brain parenchyma (Bickerstaff et al. 1952, 1956). The macroscopic appearance of these parasites is that of giant cysts associated or not with several membranes attached to each other (Fig. 3.7).
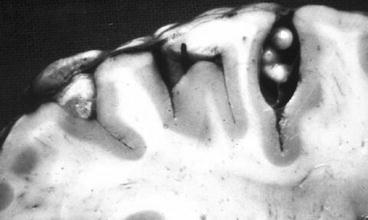
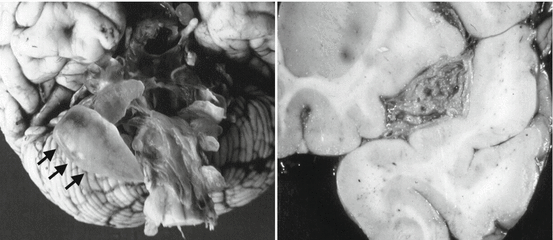

Fig. 3.6
Small subarachnoid cysticerci located in cortical sulci (From Del Brutto et al. (1998), with permission)

Fig. 3.7
Racemose form of neurocysticercosis located at the cerebrospinal fluid cisterns at the base of the brain (arrows) (left) and within the Sylvian fissure (right) (From Del Brutto et al. (1998), with permission)
Morphologic characteristics of ventricular cysticerci are varied as they may be small or large, and may or may not have a scolex. These cysts may be found freely floating within the ventricular cavities or may be attached to the choroid plexus or the ventricular wall (Bickerstaff et al. 1956; Madrazo et al. 1983; Milenkovic et al. 1982). It is generally accepted that cysticerci enter the ventricular system through the choroid plexus of the lateral ventricles but are most often located in the fourth ventricle, though they may also be located in the third or lateral ventricles as well (Fig. 3.8). This is due to the fact that when parasites reach the ventricular cavities, they have a size that allows them to follow the cerebrospinal fluid circulation pathway from the lateral ventricles to the third ventricle through the foramina of Monro and, then, from the third to the fourth ventricle through the cerebral aqueduct. By the time cysticerci have reached the fourth ventricle, most have attained a size that prevents their further transit along the CSF circulatory pathways and get trapped within this cavity (Escobar and Nieto 1972).
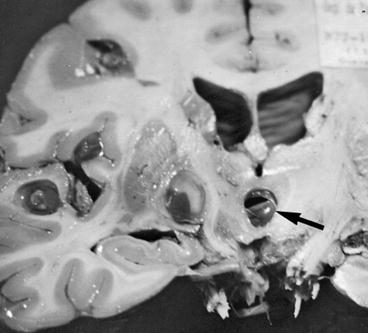

Fig. 3.8
Cysticerci located within the third ventricle (arrow). There are some additional parasites in the brain parenchyma (From Palmer and Reeder (2001), with permission)
Uncommon intracranial locations of cysticerci include the subdural space and the sellar region. In the few reported cases, subdural cysticerci have appeared as multilobulated cystic lesions showing scolices remnants, thus resembling those cysts located in the basal subarachnoid cisterns (Feinberg and Valdivia 1984; Im et al. 2005). Intrasellar cysticerci may occur as the result of direct sellar invasion by the parasites and may or may not be associated with subarachnoid or ventricular involvement (Arriada-Mendicoa et al. 2003; Del Brutto et al. 1988).
Cysticerci may also be found at the spinal level. In these cases, cysts may be located at the spinal subarachnoid space, the epidural space, or within the parenchyma of the spinal cord, the former accounting for about 80 % of cases of spinal cysticercosis (Gupta et al. 2009). Macroscopic appearance of spinal parasites is similar to those located within the cranial cavity (Kim and Weinberg 1985; Qi et al. 2011). Regarding the level of affection, most spinal cysticerci are seen in the thoracic region followed by the cervical, the lumbar, and the sacral regions, in this order (Guedes-Corrêa et al. 2006; Gupta et al. 2009). In some other cases, cysts may be scattered through all the length of the spinal cord (Jongwutiwes et al. 2011; Lim et al. 2010; Shin and Shin 2009). Through the years, there have been a number of hypothesis attempting to explain the occurrence of spinal cysticerci. While it has been suggested that cysticerci may enter the spinal subarachnoid and epidural spaces by retrograde flow through epidural vertebral veins (Sperlescu et al. 1989) and that intramedullary cysts may result from spread through the ventriculo-ependymal pathway (Trelles et al. 1970), it is most often accepted that spinal leptomeningeal cysticerci are the result of passive migration of cysts from the intracranial subarachnoid space, and intramedullary cysticerci are the consequence of hematogenous spread (De Souza-Queiroz et al. 1975). The former theory has been recently confirmed in a series of patients with basal subarachnoid cysticercosis whose spinal cords were evaluated with neuroimaging. In such study, 61 % of patients with basal subarachnoid cysticerci also have spinal subarachnoid space involvement (asymptomatic in many cases), suggesting that this form of the disease is more common than previously thought (Callacondo et al. 2012).
Cysticerci may be located in the eye, including the subconjuntival space, the anterior chamber, and the subretinal space, but only the latter should be considered as neurocysticercosis since only the retina is part of the central nervous system (Malik et al. 1968; Ziaei et al. 2011). Subretinal cysts are most often located over the macular region and are supposed to enter the eye by the posterior ciliary arteries (Bartholomew 1975; Kruger-Leite et al. 1985; Lozano-Elizondo 1983). These parasites appear flattened by ophthalmoscopic examination due to the pressure effect of the vitreous against the retina. They have a white or yellowish color with a central or eccentric, well-defined, rounded zone representing the scolex (Fig. 3.9). Ocular cysticerci may also be found freely floating in the vitreous when they move from the subretinal space through the hyaloid membrane, providing the unique opportunity to visualize in vivo the evagination movements of the parasite (Lozano-Elizondo 1983; Puig-Solanes 1982). There are some rare cases of orbital cysticerci directly involving the optic nerve (Bousquet et al. 1996; Sudan et al. 2005) or extraocular muscles (Basu et al. 2009; Sudan et al. 2007).
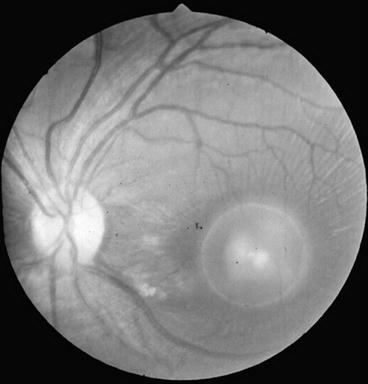

Fig. 3.9
Ophthalmoscopic appearance of subretinal cysticercus located over the macular region. The scolex is visualized as an eccentric nodule within the cyst (From Del Brutto et al. (1998), with permission)
3.3 Stages of Involution of Cerebral Cysticerci
Classical pathological studies support the concept that cysticerci are formed after the arrival of metacestodes (hexacanth embryos) to the central nervous system (Escobar 1983; Escobar and Nieto 1972). Soon after their establishment, cysticerci are viable, the bladder wall is thin and translucent, and the vesicle is filled with a clear fluid that bathes an invaginated scolex presenting a structure similar to that of the adult Taenia solium; this has been called the “vesicular stage.” According to Escobar (1983), cysticerci may remain for years in this stage or, as the result of an immunological attack from the host, may undergo a process of degeneration that ends with the dead of the parasite and its transformation into a calcified nodule. The first stage of involution of cysticerci is the so-called colloidal stage, in which the clear vesicular fluid becomes viscous and turbid, and the scolex shows early signs of hyaline degeneration. Then, the wall of the cyst thickens and the scolex is progressively transformed into mineralized granules; this stage, in which the parasite is no longer viable, is called the “granular stage” or cysticercus granuloma (Chacko et al. 2000). Then it ensues the “calcified stage,” in which cysticercus remnants appear as a solid mineralized nodule. The time parasites elapse in each of these stages has not been established, although it is accepted that considerable differences may exist among individuals (Escobar et al. 1985; Kimura-Hayama et al. 2010; Pittella 1997). Moreover, pathological studies have shown cysticerci in the four stages in the same individual, a finding that may represent cysts of different ages from recurrent infections or a single infection in which only some cysts have been attacked by the host’s immune system (Fig. 3.10).
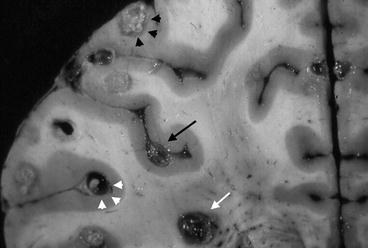

Fig. 3.10
Brain slice showing the four different stages of involution of brain cysticerci in the same person, including vesicular cyst (white arrowheads), colloidal cyst (black arrow), granuloma (white arrow), and calcification (black arrowheads) (From Sotelo et al. (1996), with permission)
It has recently been suggested that not all cysticercus granulomas are the result of degenerated vesicular cyst that had been living for years in the brain parenchyma (going first through the vesicular and colloidal stages, respectively) but represent recently established metacestodes rapidly destroyed by the host’s immune system (García et al. 2010). There are some argument favoring this alternate hypothesis of cysticerci involution, including the high rate of patients with a single cysticercus granuloma in populations exposed to low parasite loads, the low sensitivity of serological tests for the detection of anti-cysticercal antigens in patients with a single cysticercus granuloma, and the younger age of patients with a single cysticercus granuloma when compared with those with a single vesicular cyst (Del Brutto et al. 2012).
Also, recent clinical and histopathological evidence supports the fact that calcifications are not completely solid nodules and have changed previous concepts regarding the erroneous view of calcified cysticerci as totally inert lesions. Calcifications may experience periodic morphological changes related to a mechanism of remodeling. This may expose trapped parasitic antigenic material to the host immune system, causing transient inflammatory changes in the brain parenchyma that may be the cause of recurrent seizures, focal neurological deficits or recurrent episodes of headache in some patients (Del Brutto and Del Brutto 2012; Nash et al. 2004; Ooi et al. 2011; Rathore and Radhakrishnan 2012). In addition, the increasingly accepted association and probable causal relationship between calcified cysticerci and hippocampal sclerosis favors the view that calcified lesions may cause permanent epileptogenic foci that result in the occurrence of recurrent seizures (Bianchin et al. 2010; Rathore et al. 2012).
3.4 Tissue Reactions Around Cysticerci
Changes in the nervous tissue surrounding parenchymal brain cysticerci are directly related to the stage of involution of the parasites (Table 3.1). Viable (vesicular) cysts elicit little or no inflammatory reaction in the surrounding brain parenchyma. This scarce reaction is composed of lymphocytes, plasma cells, and eosinophils and is often associated with a mild astrocytic gliosis (Thomas et al. 1989). Vesicular cysts are often surrounded by a thin collagen capsule that isolates the parasite from the host and inhibits the spontaneous evagination of the scolex arresting the evolution of the cysticercus (Ostrosky et al. 1991). On the other hand, the collagen capsule surrounding degenerating cysticerci is thick and, in turn, is surrounded by an intense inflammatory reaction that often includes the parasite itself (Fig. 3.11). A dense layer of eosinophils—playing a major role in the destruction of cysticerci—is found in contact with the external membrane of the parasite (Escobar and Weidenheim 2002; Molinari et al. 1983). The surrounding brain parenchyma also shows astrocytic gliosis, microglial proliferation, edema, neuronal degenerative changes, and perivascular cuffing of lymphocytes (Chacko et al. 2000). When parasites die, the astrocytic changes become more intense than in the preceding stages and is common to see gemistocytes in the surrounding tissue. As will be discussed later, this could be the pathological substrate explaining in part the malignant transformation of glial cells that is sometimes observed surrounding parenchymal brain cysticerci (Del Brutto et al. 2000). As the gliosis becomes more intense, the edema subsides, but epithelioid cells appear and coalesce to form multinucleated giant cells which are a frequent finding around calcified cysticerci (Escobar 1983; Pittella 1997).
Table 3.1




Correlation between appearance of parasite and pathological changes in central nervous system according to stage of involution of parenchymal brain cysticerci
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







