2 Neurophysiology
General
1. How does magnesium prevent excitotoxicity in brain injury?
Magnesium readily crosses the blood–brain barrier (BBB) and blocks various subtypes of calcium and N-methyl-D-aspartate (NMDA) channels.1
2. What is the most abundant excitatory neurotransmitter in the brain?
Glutamate
3. What cellular elements compose the BBB?
Endothelial cells, astrocyte endfeet, and pericytes. The capillary endothelial cells are connected together by tight junctions (Fig. 2.1).
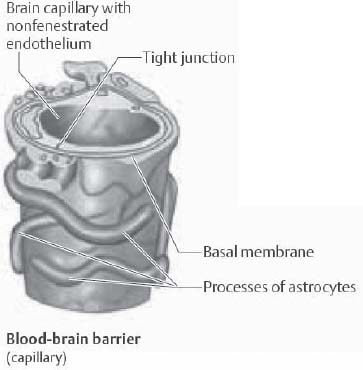
Fig. 2.1 Blood–brain barrier showing capillary tight junction. (Reprinted with permission from Rohkamm, p. 9.2)
4. What happens to platelet function after a subarachnoid hemorrhage?
It is enhanced leading to an increase in platelet aggregates in the cerebral microcirculation.3
5. What happens to cerebral blood flow immediately after a subarachnoid hemorrhage?
Decreases3
6. What is GPIIb/IIIa?
A platelet surface integrin, which is considered to be a mediator of platelet aggregation
7. What is in the dense granules of platelets?
Serotonin, adenosine triphosphate, and platelet-derived growth factor
8. It is thought that a disturbed balance between which peptide and molecule plays a major role in the development of vasospasm?
Endothelin-1 (vasoconstriction) and nitric oxide (vasodilatation)3
9. What is the name of the reaction that states that a highly reactive hydroxyl radical could be generated from an interaction between superoxide and hydrogen peroxide?
The Haber-Weiss reaction4
10. What is deferoxamine?
An iron chelator. Because intracerebral hemorrhage may result in iron toxicity to the brain, iron chelation may help to reduce brain damage in these cases.5
11. What is the proposed mechanism of action of steroid treatment in blunt spinal cord injury?
Steroid treatment in blunt spinal cord injury is controversial. Steroids (when given within 8 hours of injury) are thought to have effects on local blood flow, inhibition of immunologic injury, and free radical-mediated lipid peroxidation and neuronal damage.6
12. What is S100B and how is it related to traumatic brain injury?
S100B is a protein belonging to a multigenic family of low-molecular-weight calcium-binding S100 proteins abundant in astrocytes. After traumatic brain injury, S100B protein is released by astrocytes; this protein may be neuroprotective and/or neurotrophic.7
13. What is factor XIII and in what diseases is it deficient?
Factor XIII is an enzyme (protransglutaminase) that stabilizes the fibrin clot and is important for crosslinking fibrin in the clotting cascade. It is deficient in leukemia, liver disease, malaria, inflammatory bowel disease, disseminated intravascular coagulopathy, and Henoch-Schönlein purpura (Fig. 2.2).
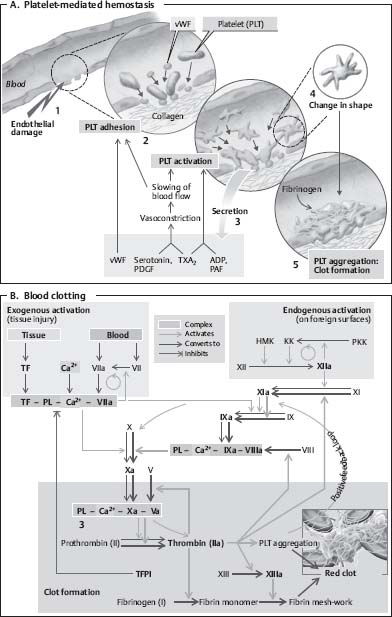
Fig. 2.2 Outline of blood clotting cascade. (Reprinted with permission from Silbernagl et al, p. 103.8)
14. What is the (intracranial) Windkessel phenomenon?
The Windkessel phenomenon is the ability of the cerebral vasculature to expand and the ability of the cerebrospinal fluid (CSF) and venous blood to translocate to accommodate arterial pulsations and provide a smooth capillary flow in the brain.9
15. What is the ischemic penumbra?
The term ischemic penumbra has been used to define a region in which cerebral blood flow reduction has passed the threshold that leads to the failure of electrical but not membrane function. The neuron is functionally disturbed, but remains structurally intact.10
16. What is the role of infiltration with local anesthetic at the beginning of a case?
Infiltration with local anesthetic prevents the activation of nociceptors during surgery and substantially lessens the need for analgesic medication.11
17. Local anesthetic molecules are composed of a benzene ring and a quaternary amine separated by an intermediate chain. Which part of the molecule determines the metabolic pathway of the drug?
The intermediate chain11
18. What is the structural unit of a gap junction?
The connexon is a proteinaceous cylinder with a hydrophilic channel and is the structural unit of the gap junction. Direct electrical communication between cells occurs through gap junctions and may be important in the pathogenesis of diseases of the nervous system including epilepsy (Fig. 2.3).12
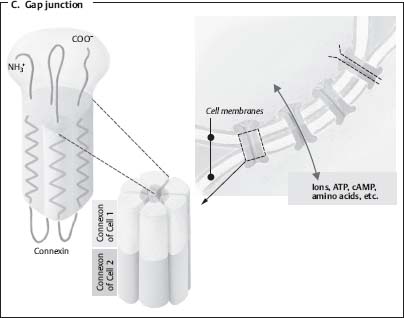
Fig. 2.3 Gap junction illustration. (Borrowed with permission from Silbernagl et al, p. 19.8)
19. What are the functions of transforming growth factor β (TGF-β)?
TGF-β is a multifunctional polypeptide implicated in the regulation of various cellular processes including growth, differentiation, apoptosis, adhesion, and motility. Abnormalities in the TGF-β signaling pathway are implicated in the development and progression of brain tumors.
20. What enzyme is inhibited by acetazolamide?
Carbonic anhydrase13
21. What is the difference between a bioactive Guglielmi detachable coil (GDC) and a platinum GDC?
The bioactive coil accelerates clot maturation and promotes the development of mature connective tissue and neointimal formation. The polymer used in bioactive coils is polyglycolic/poly-L-lactic acid (PGA/PLLA).14
22. What are matrix metalloproteinases (MMPs)?
A family of zinc-dependent endopeptidases that mediate vascular remodeling by degrading extracellular matrix components, such as collagen and elastin15
23. What is the causative mutation in Crouzon syndrome?
Crouzon syndrome is caused by mutations in fibroblast growth factor receptor 2 (FGFR2) leading to constitutive activation of receptors in the absence of ligand binding. This syndrome is characterized by premature fusion of the cranial sutures that leads to abnormal cranium shape, restricted brain growth, and increased intracranial pressure.
24. How does baclofen work?
Baclofen is an agonist of gamma-aminobutyric acid (GABA); it reduces the release of presynaptic neurotransmitters in excitatory spinal pathways.
25. What is hypsarrhythmia?
A chaotic, high-amplitude, generalized electroencephalographic pattern characteristic of infantile spasms16
26. What is “subsidence” in relation to the aging spine?
Subsidence is the loss of vertebral column height that occurs normally with aging; it may also refer to the loss of graft height after surgery. The use of dynamic plates allows normal subsidence to occur and may help bony fusion resulting in decreased incidence of construct failures.
27. What is the genetic defect in Gorlin syndrome?
Gorlin syndrome is an autosomal dominant disorder resulting from mutations in the patched (PTCH) gene that predisposes to neoplasias and widespread congenital malformations.17
28. What is rFVIIa?
Recombinant activated factor VII can help to prevent bleeding episodes especially in hemophilic patients with inhibitors to coagulation factors VIII and IX. Administration of rFVIIa (off-label) given within 4 hours of a spontaneous intracranial hemorrhage can result in reduced hematoma growth and less intraventricular extension of the hematoma.
29. What is the name of the water channel proteins of the central nervous system (CNS), which provide a major pathway for osmotically driven water movement across plasma membranes?
Aquaporins are the water channel proteins of the brain. In normal brain aquaporin 1 is expressed on the ventricle surface by the choroid plexus. The predominant water channel in normal brain is aquaporin 4, which is strongly expressed in the plasma membranes of astrocytes. Aquaporin proteins could be a therapeutic target for pharmacologic treatment of hydrocephalus.18
30. What is the most common agent used for the pharmacologic dilatation of vasospastic cerebral vessels?
Papaverine hydrochloride is a potent, nonspecific, endothelium-independent smooth muscle relaxant that produces dilatation of arteries and arterioles, as well as veins. Intraarterial papaverine is usually administered superselectively via a microcatheter positioned proximal to the spastic vessel.
31. How do intervertebral disks receive their nutrition?
Intervertebral disks receive nutrition through passive diffusion from a network of capillary beds in the subchondral endplate region of the vertebral body.
32. What is the composition of a PEEK cage?
Polyetheretherketone (PEEK) spacers have a modulus of elasticity close to that of cortical bone. PEEK is a strong polymer that is able to withstand the compressive load of the vertebral column. Its hollow center allows for packing of autologous bone.19
33. What are common areas of leptomeningeal dissemination for tumors?
The most common areas of leptomeningeal dissemination of CNS tumors are the basilar cisterns, sylvian fissures, and cauda equina, most likely because of both gravity and slower rate of CSF flow in these areas.
34. What is the resting membrane potential in myelinated peripheral nerves? In the soma?
–90 mV and –65 mV, respectively. This membrane potential is largely determined by the potential of K+, which is ~100 times more permeable than Na+.20
35. What is the mechanism of action of the botulinum toxin?
Botulinum toxin inhibits the release of acetylcholine from the presynaptic terminal and leads to loss of muscle activation. A tiny amount of botulinum toxin can be injected into muscles to weaken them (the principle behind Botox treatment) (Fig. 2.4).21
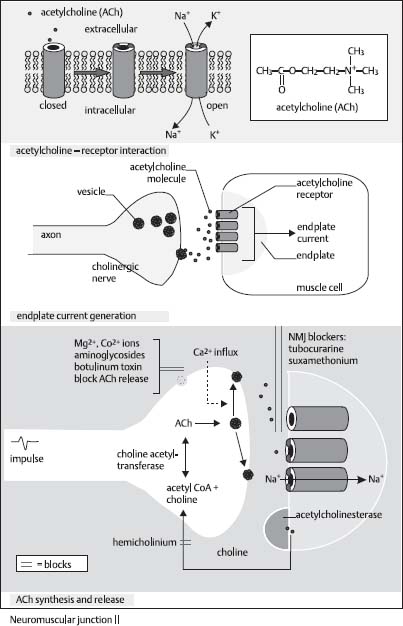
Fig. 2.4 Acetylcholine receptor and modulators. (Reprinted with permission from Greenstein and Greenstein, p. 101.22)
36. Where does the action potential in the neuron start?
At the axon hillock. This occurs because there are about seven times more voltage-gated Na+ channels there, so it depolarizes much easier than the soma (Fig. 2.5).20
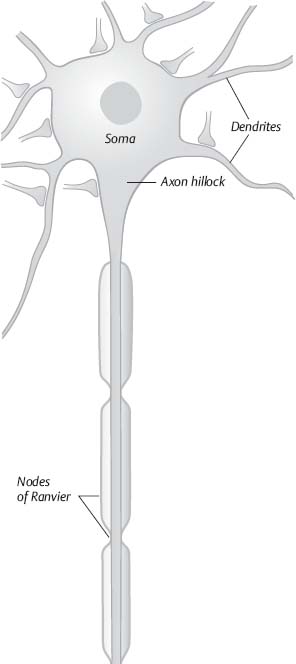
Fig. 2.5 Illustration of neuron with components. (Reprinted with permission from Silbernagl et al, p. 43.8)
37. What factors determine the velocity of propagation of an action potential?
The velocity of an action potential is affected
1. Inversely by internal resistance
2. Inversely by membrane capacitance
3. Proportionately by transmembrane resistance
4. Myelin increases transmembrane resistance and decreases membrane capacitance.20
38. What is the conduction velocity of small unmyelinated nerves? Large myelinated nerves?
About 0.5 m/s and 120 m/s, respectively20
39. How does hypocalcemia lead to tetany?
When there is less Ca2+ in the interstitial fluid, the Na+ opens sooner (at about –80 mV), so the membrane is more excitable. In other words, hypocalcemia causes a lower threshold of membrane depolarization and action potential initiation.23
40. How can hyperventilation induce seizures?
Hyperventilation causes a respiratory alkalosis, which increases the pH. Increasing pH increases the membrane excitability and can induce seizures.
41. How big is the synaptic cleft?
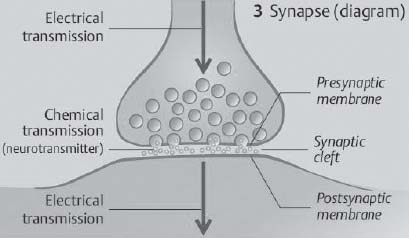
Fig. 2.6 Synapse with synaptic cleft. (Reprinted with permission from Silbernagl et al, p. 43.8)
42. What is unique about the synthesis of the neurotransmitter norepinephrine?
It is the only neurotransmitter that is synthesized within the synaptic vesicle. Dopamine α-hydroxylase is located on the membrane of the synaptic vesicle. This enzyme converts dopamine to norepinephrine (Fig. 2.7).25
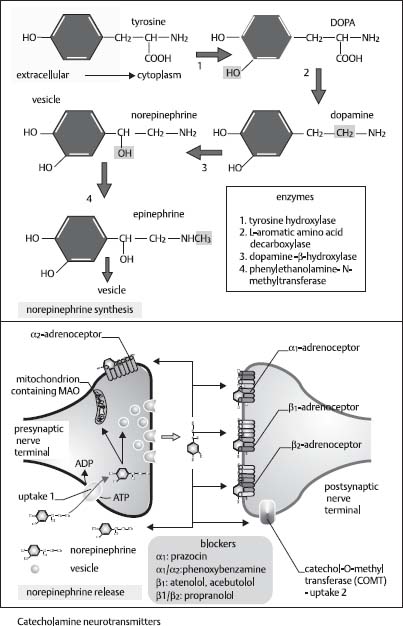
Fig. 2.7 Norepinephrine production from tyrosine (chemical steps). (Reprinted with permission from Greenstein and Greenstein, p. 111.22)
43. What are the two types of acetylcholine receptors?
1. Nicotinic receptors—located in the neuromuscular junction and preganglionic endings of both sympathetic and parasympathetic fibers
2. Muscarinic receptors—found in all postganglionic parasympathetic endings and the postganglionic sympathetic endings of sweat glands
44. What are the two main inhibitory neurotransmitters of the CNS?
GABA and glycine
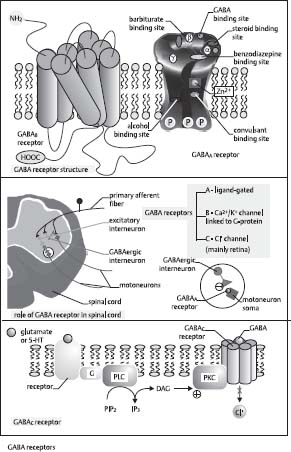
45. What is the mechanism of the GABA receptor?
The GABA receptor is made up of five subunits with a central Cl– channel (GABAc receptor). When this ligand-gated channel is activated, it increases the Cl– channel permeability and Cl– enters the cell and causes hyperpolarization (Fig. 2.8).