Fig. 1.1
(a) Basics of TMS. A large pulse of current in the external stimulating coil generates a rapidly changing magnetic field that rises to, and falls from, 1 T or more within 1 ms, and this field can penetrate the scalp and skull with little impedance. Accordingly, the electrical field it induces causes an eddy current to flow in the area of the brain beneath the coil, resulting in depolarization of axons in the cortex. If TMS is applied over the primary motor cortex, it can induce a small twitch in the target muscle, so-called motor evoked potential (MEP). (b) Mean effects of theta-burst stimulation (TBS) on MEP amplitudes in nine individuals. In these people, intermittent TBS (iTBS) produces lasting increase, while continuous TBS (cTBS) induces lasting decrease of MEP sizes compared to baseline. Modified from Huang et al. (2005) (c, d) Effects of TBS are highly variable when larger number of participants are analysed. Data plotted from 52 healthy young subjects (Modified from Hamada et al. (2013))
There is good evidence that rTMS can produce after-effects on the brain, offering potential for clinical application in variety of neurological and psychiatric diseases (Chap. 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12). These after-effects outlast the stimulation period and are usually described as “LTP-/LTD-like” plasticity depending on whether the overall effect is an increase or decrease in cortical excitability, as indexed by motor evoked potential (MEP) amplitudes (Fig. 1.1a). There are number of similarities to synaptic plasticity in animal preparations (Ziemann et al. 2008). First, the effects are likely to take place at the cortex because spinal excitability is not altered by the interventions. As with many demonstrations of synaptic plasticity in animals, in humans, the effects often evolve rapidly, yet are reversible, lasting for 30–60 min. Furthermore, it has been shown that NMDAR antagonists block the plasticity induced by some rTMS protocols (Stefan et al. 2002; Wolters et al. 2003; Huang et al. 2007). Thus, at least some forms of plasticity induced by rTMS are likely to be NMDA dependent. Synaptic effects of rTMS are also compatible with its interaction with behavioural learning (Ziemann and Siebner 2008) or recovery after stroke (Di Pino et al. 2014; Grefkes and Ward 2014). Thus, forms of rTMS can suppress (Muellbacher et al. 2001; Baraduc et al. 2004; Kang et al. 2011) or facilitate learning (Jung and Ziemann 2009). Given that synaptic plasticity is a likely substrate for learning, it has been implicitly assumed that such interference may be caused via effects on synaptic plasticity.
As in animal experiments, several protocols have been reported to induce LTP- and LTD-like plasticity (Table 1.1). Conventional rTMS refers to rTMS at fixed frequency: high-frequency rTMS at 5 Hz or higher transiently increases cortical excitability (i.e. LTP-like), while stimulation at 1 Hz decreases cortical excitability (LTD-like) (see also BOX1). Patterned rTMS involves more complex protocols, the most common of which is theta-burst stimulation (TBS) which consists of a burst of 3 pulses at 50 Hz, repeated at 5 Hz, as in slice preparations (Huang et al. 2005) (Fig. 1.1b). Another example is quadripulse stimulation (QPS) in which a burst of 4 pulses is repeated at a rate of 0.2 Hz. Depending on the interval within 4 pulses, QPS is capable of inducing either LTP- or LTD-like plasticity (Hamada et al. 2008). Paired associative stimulation (PAS) is another commonly used protocol in which electrical stimulation of peripheral nerve is repeatedly paired with TMS over the contralateral primary motor cortex. The effective median nerve-TMS interval at approx. 21.5–25 ms or 10 ms is thought to reflect the time window for development of spike timing-dependent (STDP) plasticity at cortical synapses activated by median nerve input and TMS (Stefan et al. 2000; Wolters et al. 2003). LTD-like effects are seen when the TMS-verve interval is 10 ms, whereas LTP-like effects occur at 21.5–25 ms.
Table 1.1
Summary of rTMS protocol for LTP- and LTD-like plasticity induction
Protocol | LTP-like plasticity | LTD-like plasticity |
|---|---|---|
Conventional rTMS | High frequency, >5 Hz | Low frequency, 0.2–1 Hz |
Patterned rTMS | ||
TBS | Intermittent TBS | Continuous TBS |
QPS | QPS-5 | QPS-50 |
PAS | PAS25 | PAS10 |
Although the effects induced by rTMS (see above) are consistent with modifications of synaptic plasticity, we still lack definitive proof of their origin. Similarities such as NMDA dependency do not necessarily imply common mechanisms. In addition, unlike slice experiments in which one pathway or connection is investigated, the plasticity of rTMS results from the sum of changes in a number of excitatory and inhibitory connections (Di Lazzaro and Rothwell 2014). In fact, it is possible that synaptic plasticity evoked by rTMS in one pathway may not be the same as in other pathways (Dan and Poo 2006; Feldman 2009; Collingridge et al. 2010). Even in animal experiments, LTD is easily induced in excitatory synapses of distal dendrites, while proximal synapses are prone to LTP (Letzkus et al. 2006). Furthermore, there are different types of STDP at inhibitory synapses (Feldman 2012). Another puzzling point is that it is often difficult to induce synaptic plasticity in neocortex of adult or behaving animals (Hess and Donoghue 1994; Racine et al. 1994a, b; Hess et al. 1996; Chapman et al. 1998; Trepel and Racine 1998), while it seems to be very easy to produce plasticity by rTMS in adult human brain. In behaving animals, the LTP protocol usually requires stimulation for days (Trepel and Racine 1998) or even application of a GABA-antagonist to achieve disinhibitory states (Hess et al. 1994). In contrast, cTBS using rTMS induces LTD-like plasticity in a few minutes in adult human brain (Huang et al. 2005). These data raise the question whether synaptic plasticity is solely and exclusively responsible for what we observe in intact humans. Taken together, since after-effects of rTMS result from mixture of distinct (either LTP or LTD) changes in (presumably) a number of different synaptic connections, it may be an oversimplification to describe the after-effects of rTMS as LTP or LTD-like plasticity exclusively based on MEP changes.
1.3 Variability in Response to rTMS
Ever since the introduction of rTMS (Pascual-Leone et al. 1994), it has been well recognized that the response to rTMS is highly variable. This was firstly reported in a small number of subjects with conventional rTMS (Maeda et al. 2000). Subsequent studies in larger numbers of healthy subjects have confirmed that there is a considerable variability in response to any rTMS protocol (Table 1.1) (Müller-Dahlhaus et al. 2008; Hamada et al. 2013; López-Alonso et al. 2014; Wiethoff et al. 2014). In general, the probability of producing the “expected” response may be as low as 50 %, at least as measured by effects on the MEP based on the recent studies with relatively large number of subjects (Figs. 1.1c, d, and 1.2) (see also (Horvath et al. 2014)). A number of factors have been identified to explain this variability, such as age, gender, time of day, physical activity, prior history of synaptic activity, state of cortex, interneuron networks, or even genetics (Ridding and Ziemann 2010). However, none of them accounts for a large proportion of the variation which thus must be regarded as multifactorial. It may be possible to simplify the sources of variability into two groups: intrinsic and extrinsic. Intrinsic variability may relate to factors that are impossible to modify, such as age, gender and genetics. Extrinsic variability is potentially controllable and includes factors such as state of cortex, prior history of synaptic activity, time of day, physical activity, detection of the motor hotspot, the attention level of subjects in a long experiment, etc. For example, some evidence suggests target muscle activity prior to or during rTMS intervention affects response variability. It might be possible to minimize this by a short period of complete EMG silence in target muscle prior to delivering rTMS. However, it is difficult to define a true “rest” condition. Even though participants may maintain complete silence in a target muscle, this does not guarantee that this is true of the whole motor system. In fact, even in a target muscle at rest, motor threshold can be modified when subjects change the focus of their attention (Gandevia and Rothwell 1987). This implies that the resting condition may vary depending on unavoidable fluctuations of neuronal states including attention, and thus any measure related to rest (e.g. resting motor threshold or MEP at rest) may be ill defined. Finally, it should be remembered that variation in response to rTMS may be due to variation in the ability of the test stimulus to pick up the effects. This could reflect, for example, interindividual variability in interneuron networks involved in the MEP.
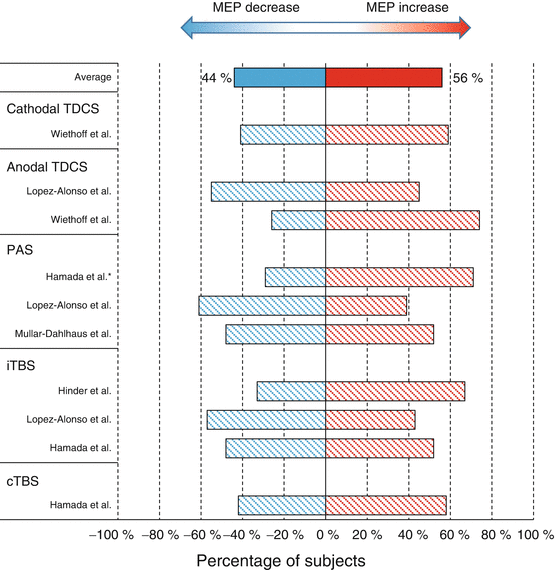

Fig. 1.2
Summary of response profile of each protocol. The bar indicates the percentage of subjects who showed MEP increase or decrease in each study. Note that this is not a meta-analysis and the studies were chosen from recent studies. This is because these include a relatively large number of subjects (more than 25 subjects) compared with the studies previously reported (see also Horvath et al. 2014). * unpublished data
Although there are problems in using MEP measurements to detect effects of rTMS, the advantage is that they provide an objective and useful way to measure cortical excitability. Apart from MEP, EEG responses to TMS (transcranial evoked potential, TEP) are a second objective read out of TMS (Massimini et al. 2005; Premoli et al. 2014). The advantage of TEP is that it is available, in principle, to any area of the brain, in contrast to the MEP which can only obtained by TMS over the primary motor cortex. However, there are no studies of range of variation in TEP measures after rTMS in different individuals.
1.4 Effects of rTMS on Behaviour
There is a good evidence that rTMS improves or facilitates the function of certain areas after brain damage or dysfunction. In fact, many clinical trials have reported favourable effects on symptoms in various neurological and psychiatric diseases, such as stroke, depression, Parkinson’s disease, pain, etc (Lefaucheur et al. 2014). However, the beneficial effects of rTMS are variable, and the results of these trials are inconsistent. The question is why does this happen?
As already mentioned, we know that the effects of rTMS on MEP excitability are highly variable, but it is not yet clear whether variability in MEPs translates directly into variability in behavioural effect. It is often tacitly accepted that this relationship exists since we select for therapy those protocols that have the “desired” effect on MEPs. However, it may be too simplistic assumption, and therefore, it is worthwhile to know whether the response to rTMS measured using MEPs predicts either (a) a person’s intrinsic ability to learn a certain task and/or (b) the effectiveness of an rTMS protocol to enhance a person’s performance in a task. For the first point, there is some evidence that MEP changes produced by rTMS do not correlate motor learning rate (Li Voti et al. 2011). The answer may be more positive for the second point. Kang et al (2011) found a negative correlation between rTMS effects on MEPs and the effects of the same rTMS protocol on motor learning (Kang et al. 2011). However, the number of subjects was small, and more information is required to answer the question with certainty. Finally, it may be important to note that the MEP only reflects activity in the large diameter axons of the pyramidal tract. These represent only about 2 % of the total tract. Thus, it is possible that at least some effects of rTMS on behaviour result from activity in other components of the tract or even activity in other tracts such as the rubulospinal, reticulospinal, cortico-cortical and cortico-subcortical pathways (Lemon 2008). In this context, it is interesting to note that MEP changes in the corticospinal system may not correlate with changes in other pathways. Thus, application of an inhibitory rTMS protocol (QPS) over left primary motor cortex (M1) reduced MEPs evoked from left M1, but did not change interhemispheric (cortico-cortical) inhibition from left to right M1 (Tsutsumi et al. 2014), suggesting that effects on cortico-cortical and corticospinal pathways differ. Future studies are required in order to predict the effects of rTMS in a clinical setting.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





