Fig. 26.1
66-year old patient with the history of colorectal cancer who presented with vertigo and dysarthry. (a) native CT shows a dense mass in the right cerebellar hemisphere with surrounding edema towards the fourth ventricle (arrow). (b) T1-weighted MRI after contrast administration shows the same lesion as solid, contrastenhancing mass. (c) T2-weighted MRI shows the lesion and highlights the hyperintense peritumoral edema (arrows). (d) Post-operative CT shows the resection cavity while the fourth ventricle is now, after the removal of the space-occupying lesion, expanded to normal size (arrow).
Treatment of Brain Metastasis
The decision whether patients should undergo surgical resection always needs careful evaluation of the individual case and informed consent of the patient. If possible, the patient`s family should be included in the decision process.
The mainstay of treatment for patients with brain metastases is complete surgical excision of the lesion. Surgery is able to (1) erase the space-occupying lesion and therefore relief the patients symptoms and, in most of the cases, improve neurological function (Stark et al. 2011). (2) Surgery enables histological examination of the tumor tissue which is essentially required for adequate adjuvant treatment. According to advances in operative techniques and neuroanesthesia, surgical complications nowadays can be reduced to a minimum and surgical procedures can be applied to a rising amount of patients including patients of advanced age (Al-Shamy and Sawaya 2009; Siu et al. 2011).
The Indication for Surgery
To date, surgery is generally warranted under the following conditions assuming that the metastases are accessible and the primary tumor is under control or unknown.
1.
In solitary or singular brain metastasis, either symptomatic or not, surgical treatment is regarded as the standard treatment option. The benefitial role of resection in addition to whole brain radiation therapy has been well documented since the early 1990s (Patchell et al. 1990; Vecht et al. 1993).
2.
Life threatening lesions require immediate surgery. This is the case in large lesions causing mass effect, finally leading to tentorial herniation. It is also an important issue in infratentorial tumors causing obstruction of the aqueduct resulting in acute, life-threatening hydrocephalus.
3.
The diagnosis is uncertain (cancer of unkown primary, CUP). Tumor resection, or biopsy in cases where tumor removal would cause unacceptable neurological deficits, enables accurate histopathological diagnosis. Remarkably, the rate of histopathological “surprises” in cases with suspected brain metastases based on clinical observations is in the range of 11 % (Al-Shamy and Sawaya 2009).
In contrast to patients with singular metastases, prospective randomized trials are lacking for the treatment of patients with multiple metastases. However, according to the recent literature and our own experience it seems appropriate to resect all lesions if technically feasible (Al-Shamy and Sawaya 2009; Siu et al. 2011). We tend to remove up to 3, maybe 4 lesions in 1–2 operations during one anesthesia in these cases. If needed, the patients head position is changed in between.
As a consequence of increasing senescence of the population, the amount of elderly patients with brain metastases is rising. There is actually no reason to generally exclude elderly patients from surgical treatment. In a systematic review, we have found that survival in patients over the age of 65, in contrast to younger individuals, is affected by the number of metastases (<=3 vs. >3) and the presence of co-morbidities. In younger patients the presence of extracranial metastases was significantly associated with reduced survival. In both groups, as expected, favorable patient performance was associated with prolonged survival (Stark et al. 2011).
The role of histopathological diagnosis in neurooncology cannot be overestimated. Modern imaging techniques can approximate the diagnosis but they can, at least up to now, not prove it. Only histopathological examination based on paraffin sections can. It is essential to obtain enough tissue for diagnosis and store it adequately. Further material might be stored in liquid nitrogen or −80° freezers for genetic testing.
Surgical Technique
Pre-operative preparation for craniotomy requires patient consent to operation and anesthesia as well as MRI before and after contrast administration. CT might be added if bone erosion is suspected, it is essential in cases with skull base involvement. MRI or CT slides are prepared for neuronavigation. Besides laboratory blood examination, we perform chest X-ray and electrocardiogram in every patient over the age of 40 years on a routine basis.
Intraoperatively, rigid head fixation is needed to prevent patient movement during the operation. All intracranial tumor operations are carried out under the microscope with a magnification of 6–40 fold. Neuronavigation is routinely applied for minimizing the access to the tumor and for resection control in large metastatic lesions which, in the end, is benefitial for the patient (Tan and Black 2007). Neuronavigation represents a 3D-computer model based on a pre-operative CT or MRI scan that is intraoperatively available. The surgeon can check the position of a pointer held to (for craniotomy planning) or into (for detection of small lesions and resection control) the patient`s head in reference to the computer model. A limitation of this technique is the fact that the intracranial structures move after opening of the skull and resection of intracranial tissue. This incidence is called brain shift.
After craniotomy, the bone flap and the dura can be inspected for possible tumor infiltration. Superficial lesions are sometimes, though not always, visible through the cortex. In deep-seated small lesions neuronavigation is extremely helpful in minimizing the access through the sulcus or the brain parenchyma. After the lesion is accessed, it is debulked before the border can be dissected in order to prevent damage to the adjacent brain. Cyst fluid can be punctured, leading to additional reduction of space-occupation. The first tumor tissue removed can be used for frozen sections for further approximation of the diagnosis. After dissection of the tumor/brain interface the metastasis is removed, either in toto, or piece by piece. Resection should be complete to reduce the risk of local recurrence. After meticulous hemostasis of the resection cavity, the dura is closed in a watertight fashion. This is important since post-operative CSF fistula is a major source of peri-operative morbidity and a significant risk factor for infection. Following dural closure, the bone flap is replaced and fixed and the wound is closed. To our experience, a wound drainage can be omitted in most of the cases. Figure 26.2 shows pre-operative MRI and intra-operative microscopic images of a superficially located brain metastasis.
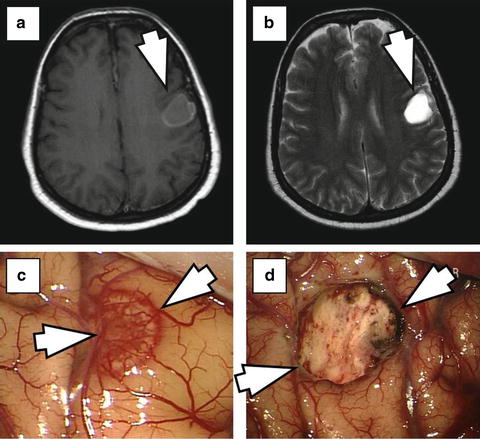

Fig. 26.2
55-year old patient with known breast cancer who presented with aphasia. (a) T1-weighted MRI after contrast administration shows a cystic ring-like contrast enhancing left temporo-parietal lesion (arrow). (b) T2-weighted MRI demonstrates cyst fluid inside the lesion (arrow). (c) Intra-operatively, after opening the dura, the lesion is visible through the cortex (arrows). (d) Intra-operative imaging after microsurgical resection shows the resection cavity.
Post-operative care
It is essential to mobilize the patient early after the operation in order to prevent thrombosis and pneumonia. In this situation, specially trained physiotherapists can effectively contribute to favorable patient performance and outcome. Depending on the medical system, the hospital stay is usually in the range of 3–7 days. The sutures are removed the 7th–10th postoperative day. Patients can wash their hair 24 h thereafter.
After obtaining the definite histopathological diagnosis based on paraffin sections further therapeutic steps can be planned (radiotherapy, chemotherapy, combined approaches).
Medical and Adjuvant Treatment
Medical treatment for brain metastases constitutes in the application of corticosteroids which can stabilize the blood–brain barrier and therefore reduced peritumoral edema. Corticosteroids are mandatory in the perioperative phase in order to prevent further brain swelling. Postoperatively, the peritumoral edema decreases and corticosteroids can be reduced.
Whole Brain Radiation Therapy
Whole brain radiation therapy in addition to surgical excision has been shown to reduce local and distant recurrence but it does not prolong survival (Al-Shamy and Sawaya 2009).
The combination of whole brain radiation therapy and stereotactic radiosurgery can also not prolong survival but again improve local control when compared to radiosurgery alone. This observation has been made in a series including 132 patients with 1–4 metastases (Aoyama et al. 2006).
Stereotactic Radiosurgery
Stereotactic radiosurgery (SRS) refers to the (usually) single time application of small collimated beams of ionizing radiation to an ill-defined intracranial mass. The most often used techniques are Gamma Knife Surgery (multiple cobalt sources) or LINAC (linear accelerator). SRS can be applied on an ambulatory basis. The technique is limited by the size of metastases of up to only 3 cm diameter.
The role of SRS as adjuvant treatment following open resection is currently under examination. In a retrospective study including 47 patients who underwent stereotactic radiosurgery (Gammy Knife) to the resection cavity after complete removal of brain metastases as well as to synchronous or metachronous metastases, Jagannathan et al. saw effective local tumor control. Herein, whole brain radiation was reserved for patients with small numbers of metastases and favorable performance, finally applied in 10/47 patients (Jagannathan et al. 2009).
SRS has also been evaluated as single treatment option for small metastases (<=3 cm in diameter) instead of surgical resection. Overall, there is a growing body of evidence that surgery is superior to SRS in these circumstances (Al-Shamy and Sawaya 2009).
Procedure in Patients with Cancer of Unkown Primary
In cases of cancer of unknown primary (CUP) gaining tumor tissue is essential for histopathological diagnosis. Histopathology can give first information concerning the underlying tumor (adenocarcinoma versus squamous cell carcinoma). Using special immunohistochemical markers, such as certain cytokeratins, the primary tumor can be targeted more precisely (Drlicek et al. 2004). In our series, 93 patients (30.1 %) presented with brain metastases as first sign of malignant disease (the far most of the patients suffered from lung cancer, see above). In a total of 8 patients (2.6 %) the primary tumor remained unknown even after diagnostic workup during the perioperative period (Stark et al. 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








