By the end of the 19th century it was recognized that signalling from one neurone to the next occurs at specialized contacts – Sherrington coined the term ‘synapse’. It took another 50 years for scientists to accept that information passes between neurones principally through the movement across synapses of chemicals and not electrical current. Today changes in chemical transmission at brain synapses are accepted as being key to the successful drug treatment, and cause, of many forms of psychiatric illness. This article focuses on general aspects of chemical transmission and describes some recent advances relevant to psychiatry that point the direction of future research.
Otto Loewi identified the first chemical neurotransmitter, acetylcholine, in 1921. Today evidence suggests that there are many tens if not hundreds of molecules in the brain that have neurotransmitter properties. These molecules include not only the three major classes of neurotransmitters—amines, amino acids and neuropeptides —but also specific purines, trophic factors, inflammatory mediators (chemokines and cytokines), lipids, and even gases. Examples of molecules that serve neurotransmitter functions in the brain are listed in
Table 2.3.4.1. This list is not exhaustive and more are likely to be discovered.
Basic principles of chemical transmission
Typically a molecule is classified as a neurotransmitter if it is localized in neurones, released from nerve terminals (and often soma and dendrites) on membrane depolarization, and exerts physiological and molecular effects through acting on postsynaptic receptors. However, the degree to which a particular molecule satisfies these criteria may vary. For example, the term ‘neurotransmitter’ was once used to cover only those molecules that exert fast synaptic effects, whereas molecules that exerted slow synaptic effects were termed ‘neuromodulators’. These distinctions are less useful today (and will not be used herein) as it is recognized that many molecules are capable of exerting both fast and slow synaptic effects, depending on the receptor activated. Moreover, it is now recognized that certain molecules transfer information at a synapse in a ‘retrograde’ direction. In this case the molecules are located in the postsynaptic neurone and, when their synthesis is activated, the molecule diffuses across the synapse to act presynaptically.
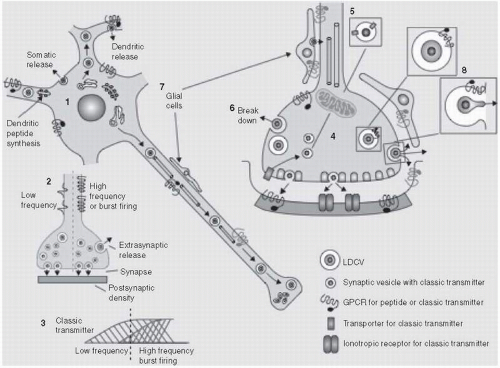
The general principles of the chemical transmission at central synapses are similar across all neurotransmitter molecules but there are often important differences between molecules especially across molecules of different size, in particular small molecule transmitters such as amines and amino acids, and peptides (
Fig. 2.3.4.1).
(1)
Small molecule neurotransmitters
Typically small neurotransmitter molecules such as amines and amino acids are synthesized at the nerve terminal by one or a few enzymatic steps and packaged in small vesicles via proton-coupled vesicular transporters, prior to release into the synapse on arrival of a depolarizing action potential. After release, the neurotransmitter diffuses across the synapse to interact with receptors on the postsynaptic neurone to trigger electrical and/or biochemical changes in the postsynaptic cell. Small molecule neurotransmitters are also released from the soma and dendrites of neurones, one purpose being to interact with presynaptic receptors that signal negative feedback to the neurone.
Once released the small neurotransmitters are selectively taken up by sodium-coupled transporters that are located in the plasma membrane of the nerve terminal or neighbouring cells (neurones or glial cells). This transport terminates transmission at the postsynaptic receptor, maintains low extracellular levels of transmitter, and allows reuse of the neurotransmitter by the neurone. Transport into the nerve terminal also presents the transmitter to catabolic enzymes to generate biologically inactive metabolites, for instance, monoamine oxidase in the case of the amine transmitters.
Neurotransmitter transporters
Advances in cloning technology has lead to new discoveries regarding the structural and pharmacological identity of transporters located on the plasma membrane, as well as vesicular transporters
located inside the nerve terminal.
(2) The latter transporters concentrate transmitters in synaptic vesicles prior to release and play a key role in determining the neurotransmitter phenotype of a neurone.
(3) A summary of plasma membrane and vesicular transporters is given in
Table 2.3.4.2.
Plasma membrane transporters
Specific plasma membrane transporters for the amine neurotransmitters, dopamine (DAT), noradrenaline (NET), 5-HT (SERT), have been identified, sequenced and, investigated in detail at the molecular level because they are the site of action of stimulants such as amphetamines and cocaine, as well as many antidepressant drugs.
(2) Indeed, the drug-binding site and the precise molecular mechanism of transporter inhibition by tricyclic antidepressant drugs have recently been revealed.
(4)Molecular cloning techniques have uncovered the genes for four transporters for the inhibitory neurotransmitter GABA; GAT-1, GAT-2, GAT-3 and BTG-1, the latter being localized primarily in the kidney. These transporters are highly homologous but pharmacologically distinct, with GAT-1 and GAT-3 being the most abundant subtypes and present on both neurones and glial cells. Although the significance of these transporters is yet to be fully understood, they display overlapping but different expression patterns in the CNS, suggesting distinct functional roles. The anticonvulsant effect of tiagabine is likely mediated by blockade of GAT-1, and there is much scope for new GABA uptake inhibitors of as yet unclear utility.
Glycine, another inhibitory amino acid transmitter, also has specific transporters located preferentially on the plasma membranes of glial cells in the forebrain (GLYT1) and neurones of the hindbrain and spinal cord (GLYT2). Interestingly, glycine is a positive allosteric co-modulator of glutamate NMDA receptors, and glycine
transport blockade may offer a means to facilitate the functioning of this receptor without incurring excitotoxic effects. The antipsychotic potential of glycine transport blockers is currently under investigation because of consistent evidence of a link between the symptoms of schizophrenia and low glutamate function.
(2)Four transporters for the excitatory amino acid neurotransmitter, glutamate, have been cloned; EAAT1 (excitatory amino acid transporter1; synonym GLAST), EAAT2 (synonym GLT1), EAAT3, EAAT4, and EAAT5.
(5) These transporters are located on both neurones (EAAT3) and glial cells (predominantly EAAT1/2) and serve to maintain low extracellular concentrations of glutamate, as well as providing a source of intracellular glutamate for metabolism. Whilst pharmacological blockade of glutamate transport increases excitotoxicity, pharmacologically enhanced EAAT expression appears to be neuroprotective.
(6)
Vesicular transporters
Vesicular monoamine transporter 2 (VMAT2) has been identified and shown to be present in neurones of dopamine, noradrenaline and 5-HT (and histamine). In addition, vesicular monoamine transporter 1 (VMAT1) is an integral protein in the membrane of secretory vesicles of neuroendocrine and endocrine cells. Reserpine is a blocker of VMAT and causes depletion of amines, and the drug’s tranquillizer effects are directly linked to this action. Acetylcholine is loaded into synaptic vesicles by a distinct transporter, VAchT.
Three homologous vesicular transporters for glutamate, VGLUT1, VGLUT2, and VGLUT3, have been cloned and characterized at the molecular level. Whilst all possess similar molecular properties, they are expressed on different neurone populations.
(3) Interestingly, VGLUT3 is found in amine-containing neurones, raising the possibility of glutamate being a co-transmitter in these neurones. The vesicular transporter for GABA is VGAT. Loss of VGAT causes a drastic reduction in release of not only GABA but also glycine, indicating that glycinergic neurones do not express a separate vesicular glycine transporter.
Neuropeptides
Following the chemical identification of the first neuropeptide Substance P in 1971, evidence has accumulated that numerous peptides play neurotransmitter roles in the brain.
(1) Some examples are shown in
Table 2.3.4.3. The neuropeptides comprise 3-100 amino acids and together with other putative signalling peptides such as growth factors and cytokines, are synthesized in the nucleus by DNA transcription followed by translation from mRNA into precursor polypeptides (
Fig. 2.3.4.1). These precursors typically undergo extensive post-translational processing that includes cleavage into smaller peptides by endopeptidases as well as other enzymic modifications. The precursor peptide usually contain an N-terminal signal sequence that directs the transport of newly synthesized protein to the lumen of the endoplasmic reticulum, and then the Golgi complex where the peptide is packaged into vesicles (so called large dense core vesicles) that are transported along the axon to the synapse. This obviates the need for neuropeptide vesicular transporters.
Proteolytic processing of a single precursor peptide often generates not one but a family of biologically active peptides, although the proteolytic steps may be tissue-specific. The opioid peptides provide one of the best worked out examples of this form of processing. Proopiomelanocortin (POMC) is a hypothalamic precursor opioid peptide whose structure contains sequences for adrenocorticotropic hormone (ACTH), α-melanocyte stimulating hormone (α-MSH) and β-endorphin. In the anterior lobe of the pituitary gland POMC is processed to form ACTH, whilst in the intermediate lobe POMC is processed to form α-MSH and β-endorphin. On the other hand, post-translational processing of the opioid precursor peptide, proenkephalin, gives rise to multiple copies of the pentapeptide metenkephalin as well as a copy of leu-enkephalin, whilst a third opioid precursor, prodynorphin, gives rise to dynorphin. In total, the 3 separate opioid peptide genes give rise to at least 18 endogenous peptides with opiate-like activity.
Multiple proteolytic enzymes have been cloned and extensively characterized, including prohormone convertases that produce striking phenotypic effects when genetically manipulated in mutant mouse models.
(7) The therapeutic utility of pharmacological manipulation of neuropeptide synthesis and degradation in the brain has yet to be realized. However, the success of inhibitors of the prohormone convertase that synthesizes angiotensin in the periphery (ACE inhibitors), for the treatment of hypertension, sets an important precedent.
Another mechanism to generate neuropeptide diversity is through alternative RNA splicing of a single gene. For example, in the case of the tachykinins alternative splicing of preprotachykinin gene A mRNA results in 3 splice variants which, after translation and post-translational processing, collectively generate the five biologically active peptides of the tachykinin family (including Substance P).
To date, there is little evidence that neuropeptides are cleared from the synapse by transporters in the plasma membrane, indicating that these particular transmitters are not recycled after release. Rather, evidence suggests that their action is terminated by peptidases that are thought to be located on extracellular membranes. Thus, replenishment of neuropeptides during high levels of synaptic activity is dependent on the proteolytic enzymes that generate the active peptides in the neurones.
A feature of most if not all neuropeptides is their co-localization with classic neurotransmitters. Some of the best examples include co-localization between GABA/dynorphin in movement control pathways (striatonigral neurones), CCK/dopamine in reward pathways (mesoaccumbens neurones), and glutamate/Substance P in pain pathways (dorsal root ganglion neurones). The functional significance of this co-localization is not fully clear but evidence suggests that peptide release requires higher frequencies of neuronal discharge than classic transmitters, and once released the neuropeptide either facilitates or opposes the function of the co-localized transmitter.
(1) In a recent example, co-localization between 5-HT and galanin in midbrain raphe neurones was investigated to reveal an action of the peptide on 5-HT feedback mechanisms. This knowledge has been exploited to develop galanin ligands that are under development as novel antidepressant strategies.
(8)