Neurotransmitters
POINT OF INTEREST
It is not uncommon in our practices for patients to announce at the initial evaluation, “Doc, I have a chemical imbalance” as though it is some sort of Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnosis. Whether a “chemical imbalance” is the source of mental illness remains to be determined, but most assuredly the manipulation of these chemicals is the bread and butter of psychiatry. They are the chemical part of the “electrochemical” communication and the focus of this chapter.
A “neurotransmitter” is technically defined by meeting the following three criteria:
The substance must be stored in the presynaptic neuron.
It must be released with depolarization of the presynaptic neuron induced by the influx of Ca2+.
The substance must bind with a specific receptor on the postsynaptic neuron.
Neurotransmitters differ from hormones by their close physical proximity of the release to the receptor—although this turns out to be less straightforward than one might imagine, as we will see in Chapter 6.
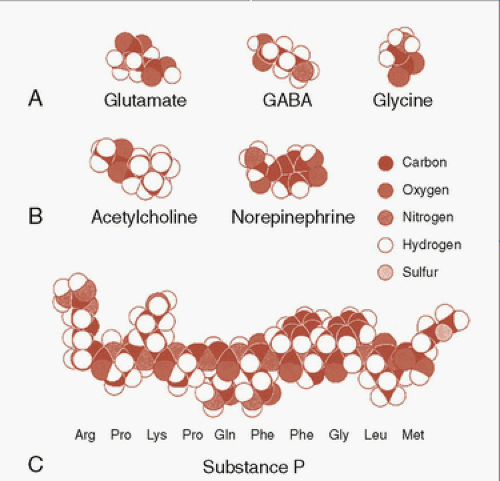
The classic neurotransmitters—the ones we frequently discuss—are small molecules designed for the ease of use. For neurotransmitters, the body needs a substrate that can be produced quickly, with ease, and be recycled—much like the daily newspaper. Figure 4.1 shows some representative neurotransmitters compared with a neuropeptide substance P. The common neurotransmitters such as γ-aminobutyric acid (GABA) and norepinephrine (NE) are small and these are constructed with elements that are easy for the body to find. This facilitates the rapid creation and release of the signals that are the essential feature of neural communication.
An extensive review of all known neurotransmitters is beyond the scope of this book. We will focus on the relevant molecules in the following three basic categories:
The classic neurotransmitters.
Neuropeptides.
Unconventional neurotransmitters.
CLASSIC NEUROTRANSMITTERS
Amino Acids
Glutamate
This is the major workhorse of the brain, with glutamate neurons making up more than half of the excitatory neurons. Without glutamate the brain does not get started or keep running. Glutamate and another excitatory transmitter aspartate are nonessential amino acids that do not cross the blood-brain barrier—an important barrier as otherwise our diet could alter our neural activity. Consequently, glutamate must be synthesized in the brain from glucose and other precursors. Glial cells assist in the reuptake, degradation, and resupply of glutamate for neurons.
POINT OF INTEREST
The body—in all its wisdom—has developed only a small number of neurotransmitters. Rather than having a billion separate molecules that each transmits a specific message, the body has a limited number of neurotransmitters that mean different things in different places. Much like the alphabet where 26 letters can create innumerable words, the body uses only a few hundred neurotransmitters to coordinate the most complex organ in the universe.
POINT OF INTEREST
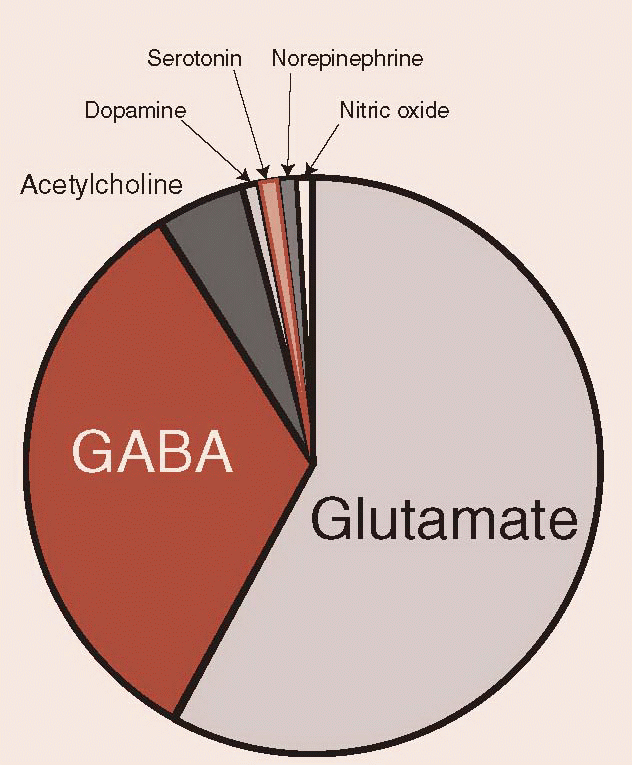
Of the classic neurotransmitters, the monoamines are the ones we typically talk about. Hanging around psychiatrists, one might imagine that the brain is predominately made up of dopamine (DA), serotonin, and NE. Amazingly, these agents are present in minor amounts in the brain. The largest number of neurons and the ones that do the heavy lifting in the brain come from the amino acid group, of which glutamate and GABA are the most prominent. The pie chart gives a very rough estimate of the relative proportion of several important neurotransmitters. The neuropeptides (discussed later) would only be a line on this chart.
|
DISORDERS
Glutamate neurons are believed to be involved in the formation and storage of memories. Changes in the receptors—termed synaptic plasticity—are well documented and seem to be involved in the physical manifestation of learning. Too much glutamate, as occurs with a stroke, is toxic to the nerve cells. Not only is the cell deprived of oxygen but also the glutamate that is released from the dying cell results in further damage. Efforts are underway to find an agent that will block the toxic effects of glutamate during ischemic events to limit the secondary damage. More recently, glutamate has been implicated as a possible culprit in the pathophysiology of schizophrenia. Postmortem studies and therapeutic trials suggest that glutamatergic dysregulation may be present in patients with schizophrenia.
γ-Aminobutyric Acid and Glycine
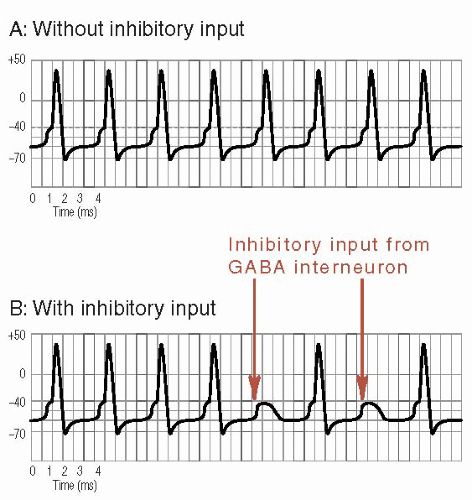
GABA is the major inhibitory transmitter in the brain and is used by approximately 25% of the cortical neurons. Glycine is the other, but less common, inhibitory amino acid. GABA puts the brakes on the brain: not enough GABA and one can have seizures. The GABA neurons are primarily the interneurons in the gray matter. They provide local constraint over too much cortical circuitry. Figure 3.7 shows an example of a GABA neuron
hyperpolarizing the receiving neuron so that it is less likely to fire an action potential.
hyperpolarizing the receiving neuron so that it is less likely to fire an action potential.
Similarly, Figure 4.2 shows how the input from a GABA interneuron quiets an overactive neuron. One can see how increasing GABAergic activity can be an effective treatment for epilepsy. More recently, increasing GABAergic activity has been used to treat insomnia, pain, and anxiety and to assist in the management of mania—all situations where too much central nervous system (CNS) activity is a component of the disorder.
Monoamines
The monoamines modulate the activity of the excitatory and inhibitory neurons. Although small in total number compared with glutamate or GABA, the monoamine neurons project widely throughout the brain. They have the capacity to “fine-tune” and coordinate the response of the major neurons.
There are two principal classes of monoamines: catecholamines (DA, NE, and epinephrine) and indoleamines (serotonin and melatonin). After being released, monoamines are degraded or reprocessed by the neuron. (Clinicians often refer to this as the reuptake pump, but neuroscientists call this the transporter, e.g., the DA transporter.) The class of enzymes in the terminal that degrades the neurotransmitters is the monoamine oxidases (MAOs). MAO inhibitors cause an increase in catecholamines (e.g., DA, NE, and serotonin), by limiting the degradation process, with well-known benefits for depression and anxiety.
Unfortunately, some food products and medications enhance the release of NE. When the MAOs are inhibited, an excessive amount of NE is released. This can result in dangerous elevations in blood pressure, which has resulted in stroke and even death in some cases.
Catecholamines
The catecholamines begin as the essential amino acid tyrosine (Figure 4.3), which must be introduced in the diet. L-DOPA (Figure 4.3A) is the molecule made famous in the Oliver Sacks story Awakenings and remains the gold standard treatment for Parkinson’s disease. The structures of DA, NE, and epinephrine are remarkably similar, yet have different functions in the brain.
Dopamine
The DA neurons constitute only about half a million of the cells in the brain—a tiny percentage out of the 100 billion total cells (Figure 4.4). Three nuclei contain the cell bodies that project the three primary branches of the DA network. The substantia nigra located in the ventral midbrain has primary projections to the caudate and putamen (collectively called the striatum). This pathway is called the nigrostriatal system or mesostriatal system. As part of the basal ganglia, this pathway is integral to voluntary movement. Parkinson’s disease is the result of a loss of DA neurons in the substantia nigra. The extrapyramidal side effects due to antipsychotic medications can induce parkinsonian symptoms by blockade of these neurons.
The cells of the ventral tegmental area, also in the ventral midbrain, project to the nucleus accumbens, prefrontal cortex, amygdala, and hippocampus. These innervations, called the mesolimbocortical DA system, are particularly dense in primates. Some writers subdivide these branches into the mesolimbic (nucleus accumbens, amygdala, and hippocampus) and mesocortical (prefrontal cortex) systems, which seems artificial as they originate from the same cell bodies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree