Figure 1.1
(a) Diffuse astrocytoma demonstrating increased cellularity and disruption of the normal orderly pattern of cells. WHO grade II astrocytomas lack the endothelial proliferation and necrosis seen in malignant gliomas. (b) WHO grade II oligodendrogliomas are characterized by uniform circular cells with a halo or “fried egg” appearance
Meningiomas
Meningiomas are typically benign, slow-growing extra-axial tumors arising from the arachnoid cap cells of the meninges. They can originate wherever arachnoidal cells are present. They are most commonly located along the falx, cerebral convexity, or sphenoid wing regions. They can also be found at the cerebellopontine angle, choroid plexus, or along the optic nerve [96]. Meningiomas account for 20–37 % of primary brain tumors with an incidence of 7.5 per 100,000 [26, 44, 96]. Meningiomas have one of the largest gender incidence differences, with a female to male ratio of 2:1. Unlike gliomas, African Americans are more commonly affected with meningiomas. Their incidence increases with age, with a median age at diagnosis of 59 years [26].
The vast majority (95–98 %) of meningiomas are WHO grade I or II [26]. Meningiomas are characterized by densely packed sheets of cells, psammoma bodies (whorls of calcium and collagen), intranuclear cytoplasmic pseudo-inclusions, and “Orphan Annie” nuclei (nuclei with central clearing from the peripheral migration of chromatin) (Fig. 1.2).
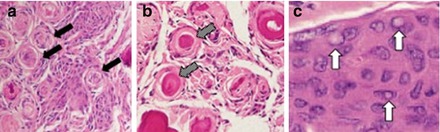

Figure 1.2
WHO grade II meningiomas show (a) densely packed sheets of cells with whorls (arrows), (b) calcifications also known as psammoma bodies (arrows), and (c) cytoplasmic inclusions (arrows)
Risk Factors
The underlying cause of low-grade gliomas in adults is largely unknown and thought to be multifactorial. Though infectious, environmental, immunological, and genetic factors have been implicated, the majority of studies lack large enough study populations to establish causation [86]. The only known modifiable risk factor for the development of low-grade gliomas is prior exposure to ionizing radiation [10]. Though speculative, hereditary factors are not yet known to play a role in low-grade glioma development, although patients with Li-Fraumeni syndrome and neurofibromatosis type 1 have an increased incidence of gliomas and form tumors at a younger age. Genetic studies suggest that point mutations of the tumor suppressor gene TP53 plays a role in the tumorigenesis of low-grade gliomas. An alteration in tumor metabolism promoting a shift to aerobic glycolysis is a principal step in the development of low-grade gliomas. Isocitrate dehydrogenase (IDH) 1 and 2 catalyze the decarboxylation of isocitrate into alpha ketoglutarate. IDH1 and IDH2 mutations are found in close to 70 % of low-grade gliomas [42, 81, 113]. The impact of these mutations on low-grade diffuse gliomas remains unclear; however, they offer an independent survival benefit in glioma patients regardless of patient age or tumor performance status.
Similar to low-grade gliomas, prior exposure to ionizing radiation is the strongest known risk factor in patients with meningioma [90]. Other modifiable factors that have been studied with inconclusive results include cigarette smoking, exogenous hormone use, personal hair dye use, microwave exposure, prior head trauma, occupational exposures, lead exposure, seasonal allergies, and cell phone use [2, 9, 12, 17, 19, 36, 37, 39, 43, 45, 48, 49, 69, 78, 82, 84, 86, 90, 96, 106]. Neurofibromatosis type 2 (NF2) caused by a mutation on chromosome 22q12 is the most common genetic predisposition associated with meningiomas. Loss of one copy of the NF2 gene occurs in up to 80 % of sporadic meningiomas and all patients with NF2. NF2 patients develop meningiomas at a higher frequency and present earlier in life [96].
Making the Diagnosis
Common Presenting Symptoms
Evaluating patients with non-malignant brain tumors begins with a detailed history. The majority of patients lack symptoms even if there is mass effect. The most common presenting symptoms for patients with low-grade gliomas are seizures (72 %), headaches (30 %), language (7 %), and sensorimotor (32 %) changes [115]. Common presenting symptoms for meningioma patients are seizures (45 %), headaches (34–41 %), dizziness (29.3 %), tinnitus (14.6 %), syncope (9.8 %), memory disturbance (4.9 %), visual disturbance (4.9 %), and trigeminal neuralgia (2.4 %) [51, 68]. Classically, headaches tend to be worse upon waking in the morning and may be severe enough to wake one from sleep. It is not uncommon for tumor-related headaches to initially be misdiagnosed as sinus, tension, or migraine headaches. Headaches related to obstructive hydrocephalus are relatively rare in non-malignant brain tumors; however, this does require urgent neurosurgical evaluation.
Seizures are the most common presenting symptom, occurring in 72–80 % of patients with low-grade gliomas and 45 % of meningioma patients [51, 115]. Seizure control is important for maintaining optimum quality of life. The use of anticonvulsant medications such as levetiracetam, lacosamide, topiramate, or phenytoin in brain tumor patients is somewhat controversial. Patients presenting with seizures should be started on anticonvulsant therapy. However, there is little data to suggest prophylactic use of anticonvulsants to reduce the risk of new-onset seizures in patients with low-grade gliomas or meningiomas [58]. Even so, it is common practice to offer perioperative anticonvulsant medications to patients with large tumors involving highly epileptogenic areas. Gross total resection is the strongest predictor of seizure freedom [30, 88]. Radiotherapy and chemotherapeutic agents such as temozolomide and alkylating agents are also effective in reducing seizure frequency in patients with tumor-associated medication-resistant epilepsy [88].
Physical Findings
The majority of people with non-malignant brain tumors have a normal neurological examination. Physical findings, when evident, are variable depending on tumor location, size, and scope of disease. Papilledema (swelling of the head of the optic nerve associated with engorgement of retinal veins) and oculomotor palsy are due to tumor mass effect and elevated intracranial pressure. Aphasia suggests involvement of cortical or subcortical language centers of the dominant frontal, temporal, or parietal lobes. Focal motor weakness can be caused by tumor invasion of the corticospinal pathway or peritumoral edema, which may be reversible with the administration of corticosteroids. The diagnosis of brain tumor should always be considered in patients who develop a new psychiatric condition.
Symptomatic Versus Asymptomatic Tumors
Non-malignant brain tumors with no clinical symptoms have a different natural history from those that are symptomatic. Incidental low-grade gliomas tend to occur more frequently in females, have smaller tumor volumes, and improved patient outcomes [77, 80]. Incidental low-grade gliomas are more likely to undergo gross total resection; however, if untreated, they demonstrate consistent radiographic growth eventually leading to symptoms over a median of 4 years [76, 77]. Incidental meningiomas fall into one of three growth patterns: no growth, steady linear growth, or exponential growth [68]. For this reason some advocate serial imaging and a brief observation period, reserving surgical intervention for patients with symptomatic lesions, documented tumor growth over time, or imaging suspicious for other pathologic lesions that mimic meningiomas [16].
Imaging
Due to its wide availability, speed, and affordability, non-contrast computed tomography (CT) is commonly the initial imaging modality for symptomatic patients with non-malignant brain tumors. Brain magnetic resonance imaging (MRI) with and without gadolinium contrast is the definitive imaging modality to assess tumor location, size, cellularity, associated cystic components, associated edema or hemorrhage, necrosis, margins and invasion into surrounding structures, vascularity, and enhancement. Radiographically, low-grade gliomas are isodense or hypodense when compared to brain tissue on CT scan and do not enhance with contrast administration. Calcifications are common in oligodendrogliomas. On MRI, low-grade gliomas are isointense to hypointense on T1-weighted imaging, hyperintense on T2-weighted imaging, and are not contrast-enhancing (Fig. 1.3). Meningiomas are hyperintense compared to brain tissue and have a broad dural attachment. On T2-weighted MRI, most meningiomas are hyperintense and are typically contrast-enhancing on both CT and MRI (Fig. 1.4). Radiographic imaging provides information about tumor localization, proximity to functional areas, mass effect, and associated edema [35]. Functional MRI and diffusion tensor imaging (DTI) shows the integrity of white matter tracts and can be used to create an operative corridor or plan for resection that minimizes risk to eloquent surrounding structures (Fig. 1.5). Metabolic MRI or magnetic resonance spectroscopy can be used to supplement information obtained from traditional morphologic MRI, particularly for differentiating radiation necrosis and residual tumor. Cerebral angiography is used preoperatively to illustrate and embolize the vascular supply to meningiomas and hemangiomas (Fig. 1.4c).
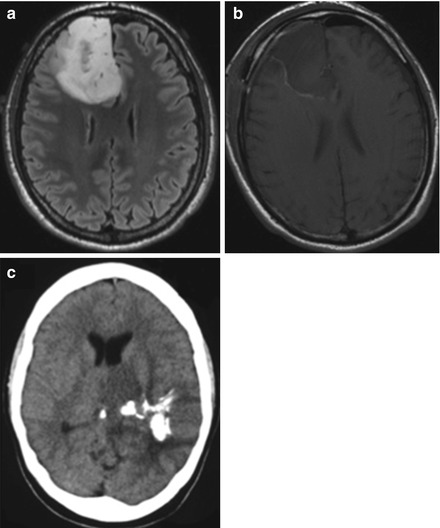
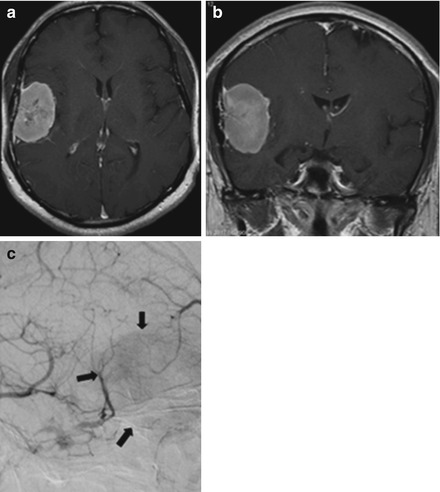
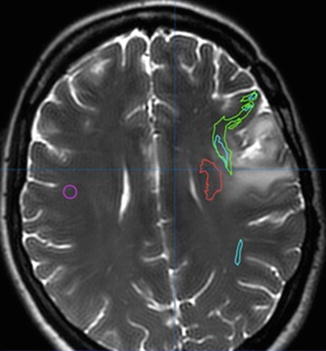

Figure 1.3
FLAIR sequence axial MRIs show right frontal diffuse astrocytoma (a) before and (b) after surgical resection. (c) Axial non-contrast head CT shows hyperdensity consistent with calcification typical in oligodendroglioma

Figure 1.4
(a) Axial and (b) coronal brain MRIs show a broad, contrast-enhancing dural-based lesion consistent with meningioma. (c) Cerebral angiography reveals dural feeders to the tumor with a “blush” of vascularity (arrows)

Figure 1.5
Axial MRI with DTI shows left frontal tumor. DTI imaging helps identify motor (red) and language pathways (green and blue) for both preoperative planning and intraoperative navigation
Treatment
The decision to offer treatment to patients with non-malignant brain tumors must balance operative risk with the natural history of the tumor and its propensity to transform and grow over time. Mounting evidence places maximal safe surgical resection alongside patient age, tumor histology, tumor performance status, and molecular markers as predictive of long-term outcome [27, 51, 61, 62, 65, 68, 97, 113]. Corticosteroids such as dexamethasone are commonly used preoperatively to reduce symptoms of mass effect and edema caused by the tumor.
Surgical Considerations and the Value of Extent of Resection
Maximum safe surgical resection is the best option to relieve symptoms, improve functional outcome, and boost long-term survival. Decisions regarding surgical approach require careful consideration of a number of key factors including: tumor size, proximity to presumed functional areas, imaging characteristics, and patient age, health, and functional status. The value of surgical resection for low-grade gliomas has been validated by a number of studies illustrating a survival benefit of 60–90 months with maximal resection [14, 38, 47, 91, 99, 105]. While some advocate initial tumor biopsy followed by watchful waiting for low-grade gliomas, several studies have suggested that this is not the best choice for long-term survival. In a large population-based series of Norwegian patients, early maximal resection was superior to biopsy and watchful waiting with respective 5-year survival rates of 74 and 60 % [47]. Furthermore, maximal resection has also been shown to delay malignant transformation with improved survival and less malignant transformation seen in patients receiving greater than 90 % extent of resection [105]. Tumor-associated epilepsy is also better managed after gross total resection [30, 114]. A small meningioma with no documented growth in an asymptomatic patient can be considered for observation [35]. This however must be balanced with the known fact that younger age at diagnosis and greater extent of resection are strong predictors of improved outcome for meningioma patients [65, 95].
The central goal of brain tumor surgery is maximizing the removal of neoplastic tissue while minimizing collateral damage to functional areas and vascular structures [29]. The timing of surgery is important in preoperative planning. The majority of patients with non-malignant brain tumors can be scheduled for elective surgery. However, patients who present with rapid deterioration due to elevated intracranial pressure or obstructive hydrocephalus require prompt intervention. For low-grade gliomas, the goal is to safely remove as much tumor as possible, as visualized by T2/FLAIR signal. The goal for meningioma resection is to remove both the tumor and its dural origin. A variety of technologies have been developed to improve surgical outcomes. Stereotactic navigation (also known as neuronavigation) is utilized to precisely localize tumor location and tailor focused craniotomies, but there is little evidence that it can improve extent of resection. Intraoperative MRI, an approach in which brain tumor resection is performed in a highly specialized surgical suite containing MRI, is another technique used to improve identification of residual tumor [59, 63, 71, 98]. The use of direct stimulation mapping to identify functional pathways is the gold standard for preserving language and sensorimotor function. Individual patient variability and pathway distortion by mass lesions makes localization of functional sites difficult by any other means [5, 18, 28, 40, 54, 72–74, 92, 102] (Fig. 1.6). Numerous studies have failed to predict the specific location of language sites using anatomical locations and functional neuroimaging (DTI and functional MRI) [1, 21, 31, 41, 60, 67, 83, 101, 110]. Using specialized neuroanesthesia, awake brain tumor surgery has proven both safe and effective, with an acceptable morbidity and mortality compared with asleep procedures [6, 22, 34, 55, 56, 89, 92, 94, 109].
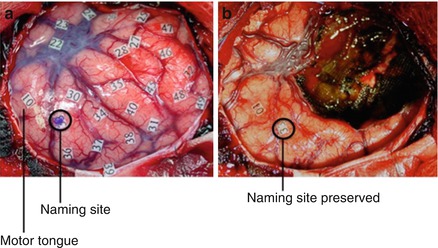

Figure 1.6
(a) Intraoperative stimulation testing allows for the identification of language, sensory, and motor sites. Numbered markers are placed 1 cm apart for testing of speech arrest, naming, and reading. (b) After tumor removal, functional sites remain intact
Following brain tumor surgery, patients are commonly observed closely in an intensive care setting with hemodynamic and neurological monitoring. Corticosteroid medications are tapered and anticonvulsants continued in patients who have a seizure history. Given the prognostic significance of extent of resection, it is standard practice for many surgeons to obtain an early postoperative MRI to evaluate for residual tumor.
Adjuvant Therapies
Although surgical resection is the foundation of brain tumor therapy, it is incapable of eliminating every tumor cell. Adjuvant radiation and chemotherapeutic regimens have been developed to treat remaining tumor cells.
Radiation
Radiation therapy can be used for select patients with recurrent low-grade gliomas to improve progression-free survival [111]. Radiation causes damage to cellular structures, inducing lethal mutations in cellular DNA and activating pathways for programmed cell death. External beam radiation can be delivered to a small precise location in a single treatment (stereotactic radiosurgery [SRS]), or in an interrupted manner (fractionated radiotherapy) that allows for repair of normal tissue between treatments. Factors that influence the use of radiation therapy include patient age (often avoided in younger patients given neurocognitive risks), speed of recurrence, volume of residual tumor after surgery, and a tumor’s molecular markers such as chromosomal translations at 1p and 19q (used to identify oligodendrogliomas, which are more sensitive to chemotherapy and radiation) [20, 46]. There is little benefit to higher doses of fractionated radiotherapy for low-grade gliomas (>45 Gy) [53]. One must balance the benefits of radiation therapy with potential late neurocognitive toxicities [4].
For convexity meningiomas, complete surgical resection of the tumor and dural attachment is often feasible. In contrast however, complete resection is often not possible for large skull-base meningiomas involving areas with high morbidity (cavernous sinus, petroclival region, optic nerve sheath, etc.). External beam radiotherapy should be considered as a safe adjuvant to surgery [93]. Long-term studies comparing adjuvant radiation with observation in patients with large skull-base meningiomas have demonstrated better tumor control after radiation therapy with 5-, 10-, and 15-year survival rates of 79–98 %, 68–93 %, and 92 %, respectively, and low morbidity (3.6 %) [24, 33, 64]. Intensity modulated radiation therapy and SRS therapy are additional options to external beam radiation for recurrent or partially resected meningiomas in patients for whom surgery is not an option.
Chemotherapy
The role of chemotherapy for non-malignant brain tumors remains unclear. Several studies exploring the use of temozolomide or procarbizine, carmustine, and vincristine (PCV) immediately after surgical resection showed little clinical efficacy [11, 13]. However, in select patients with 1p19q deletions and oligodendroglial lineage, the evidence suggests better seizure control, a progression-free survival benefit, and improved quality of life with sensitivity to PCV and temozolomide [11, 75, 77]. There is preliminary data proposing the utility of neoadjuvant temozolomide to reduce tumor volume before surgery, thereby allowing for maximal safe surgical resection [7, 8]. Chemotherapeutic agents have thus far failed to show clinical efficacy in recurrent meningioma [103]. Possible exceptions to this are currently under investigation including hydroxyurea (71 % of patients with stable disease after 2 years) and a multidrug regimen of cyclophosphamide, doxorubicin, and vincristine [15, 70, 100]. Numerous clinical trials are currently underway testing the efficacy of targeting therapies against molecular signaling pathways known to be up-regulated in non-malignant brain tumors. One promising example of this involves the use of everolimus against the AKT-mTOR pathway for patients with low-grade gliomas.
Prognosis and Long-Term Outcome
Several factors are known to be predictive of outcome for patients with non-malignant brain tumors. Negative prognostic factors for patients with low-grade gliomas include age greater than 40 years, tumor diameter greater than 4 cm, astrocytoma or oligoastrocytoma histology, tumors crossing the midline, and patients with greater than 1 cm3 of residual tumor after surgery [52, 53, 91]. These patients have a higher incidence of tumor recurrence. Patients with low-grade gliomas have a median survival ranging between 4.6 and 9.8 years and median time to malignant progression of 8.8–11.4 years when extent of resection is greater than 90 % [3, 14, 105]. Furthermore, patients with at least 90 % extent of resection have 5- and 8-year survival rates of 76 and 60 %, respectively [105] (Fig. 1.7).
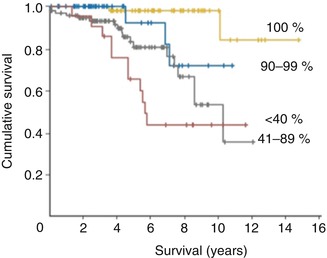

Figure 1.7
Survival curves for total, subtotal, and partial resections for low-grade gliomas (Adapted with permission [105])
The prognosis for meningioma patients is determined by the extent of surgical resection, patient age at diagnosis, and tumor grade (WHO grade I). The Simpson grading system was developed as a predictive model of 10-year recurrence based on extent of resection. Macroscopic gross total resection with excision of affected dura and bone (grade 1) offers a 9 % 10-year recurrence rate. Macroscopic gross total resection with coagulation of attached dura (grade 2) offers a 10-year recurrence rate of 19 %. Macroscopic resection without resection or coagulation of affected dura (grade 3) offers a 10-year recurrence rate of 29 %. Subtotal resection and biopsies (grades 4 and 5) offer 10-year recurrences of greater than 40 % [96, 104]. Five-year survival rates of 70–92 % and 10-year survival rates of 80–86 % have been demonstrated in patients with WHO grade I meningiomas [66, 95, 108]. Recurrence has been estimated to occur in approximately 20 % of patients with benign meningiomas but is much more common in WHO grade II and III tumors.
Conclusions
Non-malignant brain tumors are a fairly common diagnosis, and patients can live for decades after treatment. Low-grade gliomas are slow-growing tumors originating from supporting glial cells, while meningiomas grow attached to dural surfaces outside of the brain. Prior radiation exposure is the only known risk factor. Surgical resection plays a critical role. Maximal safe resection using techniques such as neuronavigation, functional MRI, DTI, and stimulation mapping allow for preservation of neurological function while reducing tumor burden. Subsequent radiotherapy can improve progression-free survival for recurrent tumors and residual disease. Targeted chemotherapeutic agents against specific pathways necessary for tumor growth are currently being investigated.
References
1.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29.PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





