Health specialists
Symptom interest, no pathology
Diagnosis
Rheumatologists
Pain, mobility, fatigue
Fibromyalgia
Physiatrists
Pain, mobility, fatigue
Regional pain
Orthopedists
Pain, mobility, fatigue
Chronic pain syndrome
Chiropractors
Pain, mobility, fatigue
Faulty spinal alignment
Infectious disease allergists and environment medicine
Fatigue, pain
Hypersensitivities
Myalgic encephalomyelitis, CFS
Multiple chemical sensitivity, SBS
Psychiatrists/psychologists
Behavior, mood, cognition
Somatoform or mood disorder
Neurologists
Headache, fatigue, behavior
Tension headache
Gynecologists
Abdominal and pelvic pain
Chronic pelvic pain, dyspareunia
Gastroenterologists
Abdominal pain, GI disorder, fatigue
Irritable bowel syndrome
Urologists
Urinary tract pain and frequency
Interstitial cystitis/irritable bladder
Dentists
Jaw pain with movement
TMJ disorder
Cardiologists
Chest pain, fatigue
Atypical chest pain
The medical field becomes more complicated when such symptoms are found in patients with a known disease. Even though the disease is successfully treated by well-established disease-modifying substances, they may remain chronically ill with unrefreshing sleep, widespread pain , tenderness, chronic fatigue, and psychological distress. These features often occur in patients with systemic lupus erythematosis and rheumatoid arthritis. Without the objective evidence for arthritic, neuroendocrine, or immunological disease pathology, the community of rheumatologists abandoned the term of ill-understood “fibrositis” and adopted the now-popular descriptive diagnosis, “fibromyalgia” (FM) or “fibromyalgia syndrome” (FMS). The formalization of criteria for the diagnosis approved by the American College of Rheumatology in 1991 [1] permitted epidemiological studies to determine the prevalence of the syndrome. Subsequently, approximately 2 % of the US population (3.4 % women, 0.5 % men) were found to be affected [2]. Those with the diffuse musculoskeletal pains and debilitating fatigue in five European countries (France, Germany, Italy, Portugal, and Spain) affected 2.9 % of the population (3.6 % women and 2.1 % men) [3], making this rheumatic disorder the second most common rheumatic ailment after osteoarthritis.
About the same time, infectious disease experts noted that pain and fatigue symptoms prevailed long after an acute infectious disease had past. They variously diagnosed such patients as having a post-infectious or post-viral syndrome, myalgic encephalomyelitis (ME), and Epstein virus syndrome. Where a sporadic cluster of such mysterious ailments appeared, diagnostic labels were attributed to geographic locations, e.g., in 1934, Los Angeles County Hospital disease also known at the time as “atypical poliomyelitis”; in 1948–1949, Akureyri disease (also called Iceland disease); and in 1955, London’s Royal Free (Hospital) disease, which subsequently was thought to be a form of mass hysteria. The absence of any verifiable evidence for any specific infectious agent or inflammatory disease pathology proved perplexing for infectious disease specialists. The lack of objective evidence for a specific virus or specific infectious disease led to a committee of advisory experts to the US Centre for Disease Control to formalize criteria. The current diagnosis, chronic fatigue syndrome (CFS), came into existence [4]. This vague descriptive label emerged in 1994 much to the chagrin of affected American patients who advocate for special recognition by government health agencies for their preferred term “chronic immune deficiency syndrome.” In the UK, the label chronic infectious neuromyasthenia encephalitis is preferred. Often, there is an overlap among the variable clinical criteria adopted by committees of the various medical disciplines so that some authors have referred to such patients as coming under an umbrella of a cluster of overlapping ailments (see Table 48.1). The overriding common feature is the absence of any known clinical features of disease or signs of any abnormalities in any radiological, hematological, histological, chemical, metabolic, or immunological tests. Nevertheless, the hunt for a viral etiology persists with the most current being the 1990 report in Science on the prevalence of a retrovirus, xenotropic murine leukemia virus-related virus (XMRV) finding in more than 60 % of the patients with CFS. Two years of several scientific reports of failures to replicate the initial findings led to a formal retraction by this journal in November 2011 [5]. A viral etiology, however, remains relevant in selected populations with post-febrile persistent chronic fatigue and diffuse myalgia. For example, following an outbreak in Toronto of severe acute respiratory syndrome (SARS), those who had survived and had no residual respiratory disease 1–3 years later had persistent diffuse myalgia, disabling fatigue , weakness with unrefreshing sleep, and anomalous increased sleep EEG alpha and the EEG sleep cyclical alternating pattern (CAP) in non-REM sleep. The possibility that the SARS coronavirus, which is known to enter the brain during the acute phase, may have a continuing adverse influence on the sleeping waking brain with associated physical and behavioral symptoms [6].
In the absence of medical disease, these people with ill-understood poor sleep quality, chronic pain, and fatigue tend to be cast into the dark chasm of vague psychiatric labels. The psychiatric diagnostic labels have changed over the course of five revisions of the American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders (DSM). They range from neurasthenia and hysterical conversion disorder to somatoform pain disorder, somatization disorder, and now the DSM-5 feckless diagnosis, “somatic symptom disorder” [7]. This chain of psychiatric labels represents a history of collective transient agreements by generations of US psychiatric committees that have applied some form of presumed scientific explanations to bodily complaints and sickness where there is no satisfactory medical or psychological explanation. Now, most of these earlier psychiatric diagnoses have been abandoned, in favor of an empirical, presumably less controversial, descriptive label. As seen in Table 48.1, the diagnostic labels adopted by various groups of health specialists are afflicted with similar scientific explanatory dilemmas.
In the prescientific evolution of knowledge, such people and their ailments were given various religious or spiritual explanations. Remedies stem from particular prayers, rituals, and/or special natural remedies. In non-Western societies, the systems of beliefs, their respective special names, and remedial methods are deemed to be features of culture-bound syndromes. For example, hwa-byung, a Korean folk illness is characterized by insomnia, pains, fatigue, weakness, gastrointestinal symptoms, and emotional distress. Chinese patients who have sleep difficulties, headaches, various pains, fatigue, and sexual dysfunction are termed shenjing shuairuo. Their society’s belief is that the affected people’s vital essence is being lost and life threatening. In the Indian society, the term is dhat, where Hindus have a similar belief in the dangers to self of the loss of essential sexual fluids. In Latin-American societies, such frightened and emotionally distressed people with similar sleep and somatic symptoms are considered to be afflicted with susto or “soul loss.” Even now in the Western society, where there is no clear medical understanding of the cause or treatment of such ill-defined symptoms, these culture-bound belief systems and methods for treating these illnesses continue to prevail in our societies. Hence, there is a large industry of purveyors of herbal substances, acupuncture, moxibustion procedures, and useless remedies of various vitamins and minerals. Where there is a lack of faith in medical explanations, there is the hope that nontraditional methods would remedy the mysterious rheumatic symptoms.
Rather than focusing on traditional rheumatic interests in joints, connective tissue, and muscles, current interest has shifted to the central nervous system as the source of hypersensitivity with application of pressure to various predesignated regions of the body and pain behavior that are readily observed by rheumatologists in examining their patients’ musculoskeletal system. Based upon experimental evidence, Moldofsky proposed the hypothesis that the sleeping/waking brain is integral to the somatic and behavioral symptoms of these ill-understood painful, fatiguing, distressing, and often disabling chronic illnesses [8]. This hypothesis gives rise to the following questions:
1.
How does the sleeping–waking brain connect to these poorly understood somatic and behavioral symptoms?
2.
Does an understanding of the disturbances in the sleeping/waking brain favorably influence the management of these suffering patients?
This chapter reviews advances in our scientific understanding of how the sleeping–waking brain is intimately connected to the widespread musculoskeletal pain , fatigue, and psychologically distressing symptoms of previously presumed to be unexplained medical illnesses, which have led to the current advances in their management.
Review of EEG Sleep, Pain, and Fatigue
The pioneering work of Moldofsky and colleagues yielded two critically important findings with regard to the relationship of sleep to widespread musculoskeletal pain and fatigue in rheumatological patients without known medical disease:
Manifestations of disturbed sleep typically comprise the experience of light and unrefreshing sleep, nocturnal restlessness with frequent awakenings, and at times, sleep-related loud snoring and breathing problems, or periodic involuntary leg movements. Typically, there is a variation in the diurnal pattern of symptoms. Upon awakening from their poor quality of sleep, patients usually experience generalized muscular stiffness, diffuse pain, and profound fatigue, which are unchanged or even increased compared to the previous evening. They report improvement in symptoms with improved energy from late morning to mid-afternoon; then, thereafter there is a downhill course in symptoms as the day progresses. The narrower the period of improvement, the more disabled is the patient. On the rare occasion, they might achieve a restful night of sleep and improved symptoms on the following day. Indeed, unrefreshing or nonrestorative sleep is closely correlated to the widespread pain and tender points in FM while psychological distress is not [12]. FM subjects with light unrefreshing sleep have diurnal impairment in speed of performance on complex cognitive tasks, which are accompanied by sleepiness, fatigue, and negative mood. Such psychological impairment could account for the functional disabilities that are encountered in a work environment and in social behavior. There may be an associated predisposition to emotional hypersensitivity resulting from distrust and insecure attachment in their personality, which is shown to be associated with the alpha-EEG anomaly during sleep [13].
These disabling behavioral symptoms are commonly associated with disturbances in the physiology of sleep [8, 9]. How these FM symptoms are linked to the subjective poor sleep quality and objective PSG measures continues to be of considerable interest in determining the pathophysiology of this illness. Early on, aspects of anomalous changes in EEG sleep physiology were identified in FM patients that were considered to be consistent with subjective poor quality of sleep. Nocturnal polysomnography (PSG) shows a prominent EEG alpha frequency (8–12 Hz) rhythm in the central EEG regions during stages 2 and slow-wave sleep (SWS) NREM sleep (see Fig. 48.1) but less frequently in patients with severely diminished or absent delta SWS [8]. As Hauri and Hawkins had noticed this alpha EEG anomaly to coincide with the delta frequency (0–<4 Hz) slow waves of SWS, they coined the term alpha–delta sleep, which they described in a small group of not depressed psychiatric patients with poorly understood illness who complained of chronic, somatic malaise, and fatigue [14]. Subsequently, Moldofsky and colleagues reported this anomaly in patients with so-called fibrositis [11], later considered by rheumatologists to be a wastebasket diagnosis because of the lack of replicated evidence of presumed inflammation of fibrous tissue. Subsequently, the symptoms were relabeled as FM or FMS. This anomalous alpha EEG sleep is seen predominantly in the frontal–central brain area in contrast to the alpha EEG frequency occurring in quiet wakefulness that is localized in the occipital region [15, 16]. Subsequently, reports of this anomalous increased alpha EEG activity in NREM sleep were reported in FM patients [8, 17–21]. Patients with FMS, however, are heterogeneous with respect to exhibiting the alpha anomaly [21]. This alpha activity in NREM sleep is not specific to FM, since it occurs in patients with other painful rheumatic diseases. Moreover, it is described to occur in patients with primary insomnia [22]. As EEG alpha-like rhythm is associated with mentation [16, 18], alpha occurring in NREM sleep may represent a vigilant arousal state that interferes with bodily restorative functions [8]. This abnormal EEG sleep feature may account for a common aspect of impaired sleep quality with symptoms of light sleep and sleep mentation with external environmental awareness and internal self-awareness of subjective discomfort. This sense of vigilance is shown to be associated with an underlying faulty personality characteristic in such people with the prominent alpha EEG sleep anomaly and physical illness which stems from a faulty interactional pattern of childhood personality development . In formal personality testing of such people with the alpha EEG sleep anomaly, most of whom have FMS, they show a pattern of overall distrust and insecurity in interpersonal relationships. Consequently, this pervasive sense of insecurity and distrust, which are ingrained in their personalities, is thought to make them difficult to accept any medical therapeutic effort that results in perpetuation of chronic physical illness behavior [13].
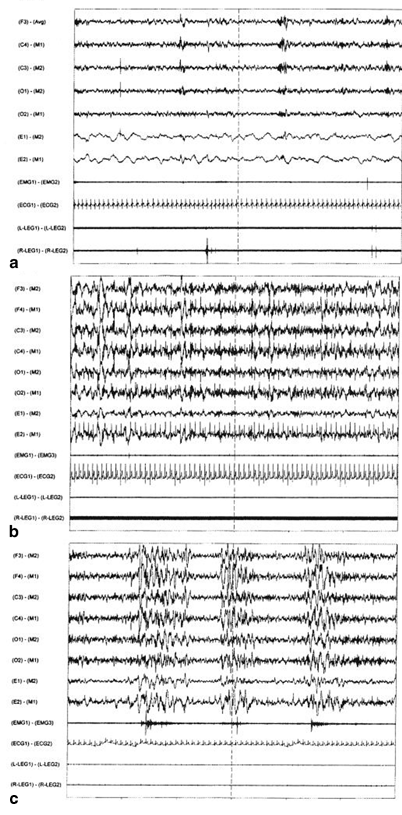

Fig. 48.1
Examples of 60-s stage 2 non-REM sleep EEG and electromyography (EMG) and electrocardiographic (EKG) recordings. a Normal, b α-EEG sleep, c. Cyclic alternating pattern (CAP) sleep EEG: subtype CAP A3 with periodic bursts of polyphasic EEG and coincident bursts of submental EMG activity. REM rapid eye movement, EEG electroencephalogram. (From [24] The Journal of Rheumatology 38:10; 2011)
A second physiological component of impaired sleep quality is thought to be a specific EEG pattern that contributes to unstable or nonrestorative sleep, a major diagnostic feature of FMS and CFS. This objective physiological feature in the sleep EEG sleep comprises a high frequency of the CAP. The CAP phenomenon occurs variably throughout NREM sleep characterized by a frequency of approximately 20–30 cycles per second (see Fig. 48.1). The specific type of CAP is reported to reflect a measure of relative stable–unstable sleep. In patients with FMS and CFS where unstable or nonrestorative sleep is commonly reported, there is an increase of CAP activity. The sleep EEG dominant rhythm of CAP A1 and no significant ancillary increase in autonomic or peripheral EMG activity, as seen in CAP A2 and increased in CAP A3, is associated with sleep stability that is commonly found in young asymptomatic healthy people. In the subgroup CAP A2 and CAP A3, there is physiological evidence for progressive increases in sleep instability [23–25]. This high amount of CAP correlates with the severity of pain as measured by the number of tender points in FMS [23]. The EEG CAP A2 and especially CAP A3 are associated with pathological sleep characterized by physiological evidence of motor and autonomic nervous system abnormalities, e.g., periodic limb movement (PLM) and sleep apnea disorders [24, 26]. Such clinically significant periodic PSG sleep disorders are found in a large sample of US patients with FMS. In this multicenter PSG study of more than 200 FMS patients, 94 % were female, with a mean body mass index of about 30 who had FMS for more than 5 years. Approximately 15 % had moderate to severe apnea–hypopnea respiratory index and 20 % had PLM disorder [24]. Their sedentary behavior with resultant weight gain and obesity may have promoted the sleep apnea disorder and the coincident CAP A2 and A3 EEG unstable and nonrestorative sleep .
Another feature of sleep EEG disturbance, which is reported in FMS, is a decrease in stage 2 EEG sleep spindles [27]. The presence of EEG sleep spindles, a characteristic of this stage of NREM sleep, is hypothesized to be a physiological feature of sleep stability. This hypothesis is supported by the shorter duration of stage 2 sleep periods, which is normally dominated by CAP A1. In addition, there is increased frequency of EEG sleep stage shifts [28], increased sleep awakenings, and decreased sleep efficiency (ratio of time asleep/time in bed) [23, 29].
Moreover, there is a reduction of SWS in FM patients [11, 19–21, 29]. One such study showed that decreased SWS is correlated with high-phasic EEG alpha rhythm [21]. The finding of reduced SWS in FMS patients also coincides with nocturnal neuroendocrine abnormalities of increased nocturnal cortisol levels and decreased release of growth hormone (GH) [30]. Quantitative studies suggest that the duration of SWS serves to regulate homeostasis and is correlated with the amount of prior waking. That is, SWS increases following extended wakefulness and is decreased during nocturnal sleep following a daytime nap containing SWS [31]. This decreased amount of SWS in FM might indicate an impairment of the SWS homeostatic drive.
Some sleep abnormalities in FM patients have not been consistently reported. In some studies, for example, FM patients are reported to be not significantly different from controls with respect to alpha sleep EEG activity or the duration of SWS [28, 32]. It is unclear whether these discrepancies result from heterogeneity of sleep pathology in FM patients, technical considerations such as differences in scoring of sleep EEG alpha anomalies, or the night-to-night variability in many sleep measures, including SWS [33].
Relationship Between Sleep and Pain Processing
Epidemiological Studies
Among patients with FMS, poor sleep quality is shown to be a key in the vicious cycle of feeling unrefreshed after sleep, morning aching/stiffness, fatigue, and dyscognition. Several prospective clinical studies report a correlation between poor sleep quality and FM symptoms. A statistical path analysis of a large population of FM patients found that a night of increased sleep disturbance correlates with increased pain, which predicted poorer physical functioning and subsequently greater depression [9]. In another study, poor sleep quality mediates the impact of increased pain upon fatigue [34]. These studies extend previous findings that a night of poor sleep is followed by a day of increased pain [35]. Moreover, after accounting for the effects of positive and negative events and pain on daily mood ratings, sleep duration and quality are prospectively related to affect and fatigue. Inadequate sleep has a cumulative effect on negative affect and prevents mood recovery from days with a high number of negative events [36].
Clinical Experimental Studies
As described above, Moldofsky and colleagues were the first to attribute functional disturbances in the sleeping–waking brain to the etiological core of promoting FM symptoms. In 1975 and 1976 publications, they showed that by interrupting SWS with auditory stimuli, an FM-like state including variable aching, fatigue, and increased sensitivity to pain pressure could be induced in healthy normal nonathletic subjects [11, 37]. They also showed in a small pilot study that under similar experimental prospective sleep and symptom test circumstances, several members of the University of Toronto track team did not experience such pain and fatigue symptoms. This preliminary finding suggests that aerobic physical fitness is involved in preventing or reducing the emergence of these symptoms. Since then, other researchers have confirmed that selective deprivation or disruption of sleep induces increased bodily sensitivity, decreased pain threshold, and reports of nonrestorative sleep [8, 38–42]. One study involving presumably fit US military men found a reduction of pain threshold, albeit not significantly different from controls [42]. Another experimental clinical study of sleep deprivation does not alter somatosensory (nonnociceptive) functions [39]. Furthermore, the effects of interruptions of SWS increases sensitivity to both painful and nonpainful stimuli, such as bright light , loud sounds, and strong odors [40]. In this study, selective sleep deprivation of middle-aged, sedentary women (similar to the major demographic population of FM patients) also produced impairment in diffuse noxious inhibitory controls (DNIC), similar to that observed in FM patients [40]. Recovery after sleep deprivation results in increased amounts of SWS and normalization of pain threshold [8]. The importance of SWS in maintaining homeostatic properties of the CNS is purported to involve downregulation of synapses [43, 44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





