Dog
Blunt tip electrodes
Electrode exposure [mm]
Separation distance [mm]
Voltage [V]
Total pulses
1
Single
7 and 5.3
8
1,600
9 × 10
2
Dual
5
5
1,000
9 × 10
3
Dual
5
5
500
9 × 10
4 – Control
Dual
5
5
1,000
9 × 10
Dual
5
10
2,000
9 × 10
5 – Control
Dual
5
5
0
0
One control animal (Dog 4) was treated at a higher voltage to evaluate the upper safety limit of the procedure by delivering ~4.5× more energy than in Dog 2 (Ellis et al. 2011). In this animal, two lesions were created using the dual electrode configuration at applied voltages of 1,000 and 2,000 V. The last animal was used as a sham control to examine the physical effects of electrode insertion without pulse delivery. Non-energized electrodes were advanced into the brain and maintained in place for approximately 30 s, the time required to deliver the IRE pulses in the other animals.
Neurologic, Imaging, and Histopathological Evaluation
After the IRE procedure, the animals were evaluated and treated in the standard fashion for post-craniectomy canine patients. There was no significant deterioration in neurologic ability or coma scale scores from baseline evaluations. The animals were able to ambulate and eat within 10 h of the procedure. No seizures were observed. Analysis of the intra-operative ultrasound obtained for each animal revealed a clearly demarcated hypoechoic zone with hyperechoic rim within the targeted brain parenchyma which was consistent with results in other organs (Lee et al. 2007; Appelbaum et al. 2012; Schmidt et al. 2012).
MRI examinations performed immediately post-operatively revealed fluid accumulation within the ablation sites and a focal disruption of the BBB (Fig. 15.1). These images also show that the IRE ablation zones were sharply demarcated and iso- to hypointense on T1-weighted sequences, hyperintense on T2-weigthed sequences, and markedly and contrast enhancing following intravenous administration of gadolinium.
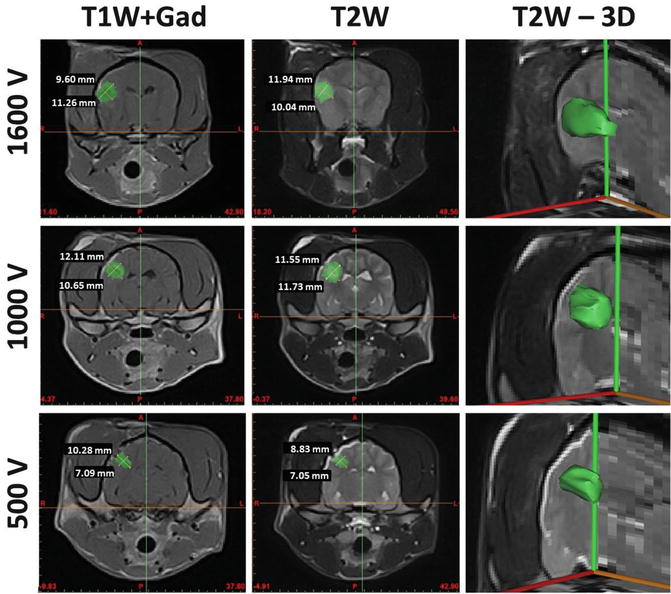

Fig. 15.1
MRI characteristics of focal brain ablations induced by IRE. Lesions (green shading) are well demarcated from surrounding brain parenchyma, homogenously T2 hyperintense, and markedly enhanced on TIW post-contrast images. There is a positive correlation between lesion size and the applied voltages
The IRE lesion in the brain of Dog 1 was more superficial than the lesions in the other animals, due to the depth of insertion of the electrodes. In this animal, a single probe was inserted parallel to the surface of the brain at a depth of 2 mm. Grossly visible brain edema and surface blanching of the gyrus overlying the inserted electrode were observed within 2 min of completion of the IRE procedure. This edema resolved completely following intravenous administration of 1.0 g/kg of 20 % mannitol. Because of these effects, the subsequent animals were treated with a smaller dual probe configuration with electrodes inserted perpendicular to the brain surface at a 7-mm depth. Brain edema and surface blanching were not observed during treatment of the remaining dogs.
The microscopic lesions from the histopathology correlated well with the gross appearance and MRI sequences in dogs 1–3. A histological comparison between the sham control and dog 3 revealed that the isolated effect of electrode insertion was limited when compared to the IRE lesion. Histopathologic sections also demonstrated that the IRE lesions have a sub-millimeter line of demarcation between areas of necrosis and normal brain. The areas of treatment were represented by foci of malacia and dissociation of white and grey matter. Small perivascular hemorrhages were present although there was sparing of major blood vessels. High-voltage pulses in dog 4 were associated with non-selective coagulative necrosis of all tissues within the treatment field, resulting in lacunar infarction secondary to arterial thrombosis. Moderate diffuse perivascular and intraglial edema, reactive gliosis, as well as death of neuronal and glial cells were also observed. The treatment area was moderately infiltrated with mixed inflammatory cells, including neutrophils, macrophages, plasma cells, and small lymphocytes. Smaller lesions were observed when decreasing the voltage between dogs. As a result, customizing the pulse parameters should allow the ablation of volumes with varying sizes and shapes.
The ablations were confirmed with histopathological analysis, revealing a sub-millimeter boundary between the necrotic and normal brain. Reconstructed lesion volumes of 1.655, 0.599, and 0.258 cm3 were calculated from the post-operative T2W MRIs using open source image analysis software (OsiriX, Geneva, Switzerland). The accuracy of the computed lesion volumes was limited to the interval between the MRI scans (2.5–3.0 mm). It is important to note that the volumes of the lesions were reconstructed from MRIs taken within 60 min after pulse administration, so the observed ablation volume is likely to be that resulting from immediate IRE induced cellular necrosis (Garcia et al. 2011b). This means that any additional cellular death resulting from late-onset apoptosis may not be taken into account in the electric field correlation (Garcia et al. 2011b). Our results support the hypothesis that IRE can be used safely in the brain and that lesion volume can be correlated with applied voltage. In this canine study, as in other studies of soft-tissue organs, IRE associated edema developed following treatment. Although the vasogenic edema observed on the MRI of dogs in this study was not associated with any clinical deterioration, it is a cause of concern. Brain edema after IRE should be anticipated and treated with perioperative corticosteroids.
IRE may offer advantages over surgical resection for selected brain tumors. The small electrode size makes the procedure minimally invasive and adaptable to virtually any neuroanatomic location with existing stereotactic guidance systems. IRE creates a sharply delineated volume of ablated tissue with sub-millimeter resolution that may make it suitable for treating deep-seated, well-circumscribed brain tumors. The minimal heat generation during treatment and sparing of major blood vessels may also make it appropriate for tumors adjacent to, or enveloping critical vascular structures. The following two sections in the chapter describe representative treatment planning procedures and clinical applications of IRE for the treatment of canine patients with spontaneous brain cancer.
Treatment Planning
Some of the advantages of IRE over other focal ablation techniques are that the treated regions are highly predictable and the technique does not depend on thermal changes to achieve tissue death (Al-Sakere et al. 2007; Ahmed et al. 2011). The extent of IRE is determined by the impedance distribution of the tissue as well as the tissue type, electrode-tissue geometry, and pulse parameters including strength, duration, number, shape, and repetition rate. However, for a given set of pulse conditions, the primary parameters affecting the degree of electroporation are the tissue type and the local electric field distribution (Edd and Davalos 2007). Therefore, the electric field distribution must be determined in order to design effective protocols for IRE procedures. Furthermore, to verify that specific protocols do not induce thermal damage due to excessive Joule heating, the temperature distribution can also be calculated from the electric field distribution and the thermal properties of the tissue. Knowledge of the electric field and temperature distribution enables researchers and physicians to reliably predict the results of an IRE procedure and minimize damage to surrounding healthy tissue. This insight enables surgeons to plan and optimize the electrode configuration and pulse parameters to:
1.
Perform IRE treatment planning using medical images
2.
Minimize applied voltages in order to reduce charge delivered
3.
Avoid inducing thermal damage due to excessive Joule heating
4.
Reduce treatment time, invasiveness, and number of procedures
5.
Ensure coverage of the entire tumor, especially when multiple applications are needed
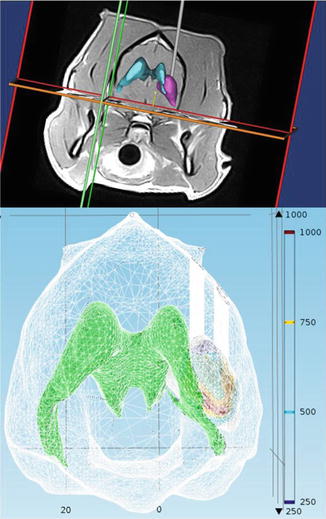
In this section we outline the IRE treatment planning procedures for canine patients with brain cancer. Specifically we describe the tissue segmentation, volumetric meshing, and finite element modeling used for therapeutic planning prior to surgical procedures.
Segmentation and Meshing of Tissue Components
Mimics 14.1 image analysis software (Materialise, Leuven, BG) is used to segment the brain tumor geometry from normal brain tissue components including the ventricles and the white and gray matter. The tumor is traced in each of the two-dimensional (2-D) diagnostic MRI, CT, or any other DICOM format imaging modalities according to intensity values. A three-dimensional (3-D) solid representation of the tumor volume, the ventricles, and the brain tissue is then refined and exported to 3-matic version 6.1 (Materialise, Leuven, BG) in order to generate a volumetric mesh for the computational models. The refined volumetric meshes are then imported into a finite element modeling (Comsol Multiphysics, v.4.2a, Stockholm, Sweden) software in order to simulate the physical effects of the electric pulses in the tumor and surrounding normal brain tissue.
Finite Element Modeling of Electric Field Distribution
The methods used to generate the electric field distributions in tissue are similar to the ones described by Edd and Davalos (2007). The electric field distribution associated with the electric pulse is given by solving the Laplace equation:
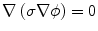
where σ is the electrical conductivity of the tissue and 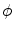 is the electrical potential (Edd and Davalos 2007). Boundary conditions most often include surfaces where electric potential is specified, as in the case of a source or sink electrode, or surfaces that are electrically insulating, as on the free surfaces of the tissue, for example. The electrical boundary condition along the tissue that is in contact with the energized electrode is
is the electrical potential (Edd and Davalos 2007). Boundary conditions most often include surfaces where electric potential is specified, as in the case of a source or sink electrode, or surfaces that are electrically insulating, as on the free surfaces of the tissue, for example. The electrical boundary condition along the tissue that is in contact with the energized electrode is 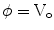 . The electrical boundary condition at the interface of the other electrode is set to ground. The remaining boundaries are treated as electrically insulating:
. The electrical boundary condition at the interface of the other electrode is set to ground. The remaining boundaries are treated as electrically insulating:
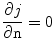
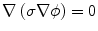
(15.1)
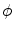 is the electrical potential (Edd and Davalos 2007). Boundary conditions most often include surfaces where electric potential is specified, as in the case of a source or sink electrode, or surfaces that are electrically insulating, as on the free surfaces of the tissue, for example. The electrical boundary condition along the tissue that is in contact with the energized electrode is
is the electrical potential (Edd and Davalos 2007). Boundary conditions most often include surfaces where electric potential is specified, as in the case of a source or sink electrode, or surfaces that are electrically insulating, as on the free surfaces of the tissue, for example. The electrical boundary condition along the tissue that is in contact with the energized electrode is 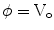 . The electrical boundary condition at the interface of the other electrode is set to ground. The remaining boundaries are treated as electrically insulating:
. The electrical boundary condition at the interface of the other electrode is set to ground. The remaining boundaries are treated as electrically insulating: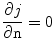
(15.2)
The models are fully defined and readily solvable using numerical methods once an appropriate set of boundary conditions, initial conditions, and physical properties of the tissue are defined. The computations are performed with a commercial finite element package (Comsol Multiphysics 4.2a, Stockholm, Sweden). The analyzed domain extends far enough from the area of interest (i.e. the area near the electrodes) that the electrically insulating boundaries at the edges of the domain do not significantly influence the results in the treatment zone.
The numerical models have been adapted to account for a dynamic non-linear tissue conductivity that occurs as a result of electroporation and redistributes the electric field during the treatment (Garcia et al. 2010, 2011a, b; Neal et al. 2012). The significant non-linear changes in the electrical conductivity of treated tissues occur because of cell membrane defects that facilitate the flow of ions and current through cells and are necessary in order to accurately represent IRE treatments. The dynamic conductivity, 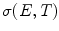 , is a function of the electric field (E) and temperature (T) of the tissue during the IRE treatment. In tissue and tumors, this increase in conductivity is approximately 3×–6× the baseline conductivity when fully electroporated (Ivorra et al. 2009; Neal et al. 2012) and needs to be determined for normal and pathologic brain tissue components. Based on the tumor dimensions and computational simulations, IRE pulse parameters are determined to ensure coverage of the entire tumors and minimize damage to the surrounding healthy tissue. The resulting electric field distributions from these parameters can be visualized in Fig. 15.2. We are currently evaluating the electric field threshold in a clinical IRE study of canine patients with MG and are using the method proposed by Neal et al. (2012) to determine the non-linear conductivity function used in the finite element simulations. The promising clinical results that we have achieved in these patients suggest that our simulations are correct and that the electric field threshold used is capable of killing the tumor tissue.
, is a function of the electric field (E) and temperature (T) of the tissue during the IRE treatment. In tissue and tumors, this increase in conductivity is approximately 3×–6× the baseline conductivity when fully electroporated (Ivorra et al. 2009; Neal et al. 2012) and needs to be determined for normal and pathologic brain tissue components. Based on the tumor dimensions and computational simulations, IRE pulse parameters are determined to ensure coverage of the entire tumors and minimize damage to the surrounding healthy tissue. The resulting electric field distributions from these parameters can be visualized in Fig. 15.2. We are currently evaluating the electric field threshold in a clinical IRE study of canine patients with MG and are using the method proposed by Neal et al. (2012) to determine the non-linear conductivity function used in the finite element simulations. The promising clinical results that we have achieved in these patients suggest that our simulations are correct and that the electric field threshold used is capable of killing the tumor tissue.
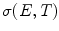 , is a function of the electric field (E) and temperature (T) of the tissue during the IRE treatment. In tissue and tumors, this increase in conductivity is approximately 3×–6× the baseline conductivity when fully electroporated (Ivorra et al. 2009; Neal et al. 2012) and needs to be determined for normal and pathologic brain tissue components. Based on the tumor dimensions and computational simulations, IRE pulse parameters are determined to ensure coverage of the entire tumors and minimize damage to the surrounding healthy tissue. The resulting electric field distributions from these parameters can be visualized in Fig. 15.2. We are currently evaluating the electric field threshold in a clinical IRE study of canine patients with MG and are using the method proposed by Neal et al. (2012) to determine the non-linear conductivity function used in the finite element simulations. The promising clinical results that we have achieved in these patients suggest that our simulations are correct and that the electric field threshold used is capable of killing the tumor tissue.
, is a function of the electric field (E) and temperature (T) of the tissue during the IRE treatment. In tissue and tumors, this increase in conductivity is approximately 3×–6× the baseline conductivity when fully electroporated (Ivorra et al. 2009; Neal et al. 2012) and needs to be determined for normal and pathologic brain tissue components. Based on the tumor dimensions and computational simulations, IRE pulse parameters are determined to ensure coverage of the entire tumors and minimize damage to the surrounding healthy tissue. The resulting electric field distributions from these parameters can be visualized in Fig. 15.2. We are currently evaluating the electric field threshold in a clinical IRE study of canine patients with MG and are using the method proposed by Neal et al. (2012) to determine the non-linear conductivity function used in the finite element simulations. The promising clinical results that we have achieved in these patients suggest that our simulations are correct and that the electric field threshold used is capable of killing the tumor tissue.