abdomen with the knee flexed. Passive extension of the leg is limited by pain when meningeal irritation is present.
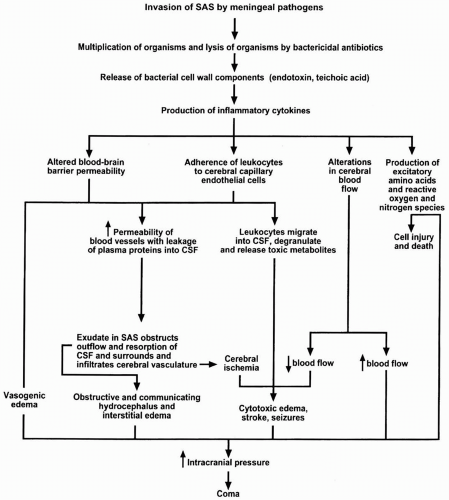
number of predisposing conditions that increase the risk of pneumococcal meningitis in the older adult, the most important of which is pneumococcal pneumonia. Due to the emergence of penicillin- and cephalosporin-resistant Streptococcus pneumoniae, empiric therapy of community-acquired bacterial meningitis in the older adult should include a third- or fourth-generation cephalosporin, vancomycin, and acyclovir. Acyclovir is added to the initial empiric regimen because herpes simplex virus encephalitis is the leading disease in the differential diagnosis. Ampicillin should be added to the empiric regimen for coverage of L. monocytogenes in older adults. In hospital-acquired meningitis and, particularly, meningitis following neurosurgical procedures, staphylococci and gram-negative organisms, including P. aeruginosa, are the most common etiologic organisms. In these patients, empiric therapy should include a combination of vancomycin and ceftazidime, cefepime, or meropenem. If a third-generation cephalosporin is chosen for empiric therapy of meningitis in neurosurgical patients and in neutropenic patients in whom P. aeruginosa is a possible meningeal pathogen, ceftazidime should be substituted for ceftriaxone or cefotaxime because ceftazidime is the only third-generation cephalosporin with sufficient activity against P. aeruginosa in the CNS. Once the organism has been identified by Gram’s stain or by bacterial culture of CSF and the results of antimicrobial susceptibility tests are known, antimicrobial therapy can be modified accordingly (Table 26-2). Some strains of pneumococci are sensitive to penicillin, but in clinical practice, few physicians use penicillin to treat pneumococcal meningitis. A CSF isolate of S. pneumoniae is considered to be susceptible to penicillin with a minimal inhibitory concentration (MIC) <0.06 µg/mL, to have intermediate resistance when the MIC is 0.1 to 1.0 µg/mL, and to be highly resistant when the MIC is >1.0 µg/mL. Isolates of S. pneumoniae that have cephalosporin MICs ≤0.5 µg/mL are considered sensitive to the cephalosporins (cefotaxime, ceftriaxone, cefepime). Those with MICs equal to 1 µg/mL are considered to have intermediate resistance, and those with MICs ≥2 µg/mL are considered resistant (11). For meningitis due to pneumococci with cefotaxime or ceftriaxone MICs of 0.5 µg/mL or less, treatment with cefotaxime or ceftriaxone is usually adequate. If the MICs are ≥1 µg/mL, vancomycin is the antibiotic of choice. Patients with S. pneumoniae meningitis should have a repeat lumbar puncture performed 24 to 36 hours after the initiation of antimicrobial therapy to document sterilization of the CSF unless findings on the neurologic examination are worrisome for a risk of herniation. Use of intraventricular vancomycin should be considered when intravenous vancomycin fails to sterilize the CSF after 24 to 36 hours of therapy. The intraventricular route of administration is preferred over the intrathecal route because adequate concentrations of vancomycin in the cerebral ventricles are not always achieved with intrathecal administration. Intrathecal administration of vancomycin is safe and is not associated with a risk of seizure activity. Cefepime is a broad-spectrum fourth-generation cephalosporin that is increasingly seen on the medication charts of hospitalized patients. Cefepime has in vitro activity similar to that of cefotaxime or ceftriaxone against S. pneumoniae and greater activity against Enterobacter species and P. aeruginosa. The dose of cefepime is 3 g intravenously every 8 hours in adults. In clinical trials, cefepime has been demonstrated to be equivalent to cefotaxime in the treatment of pneumococcal meningitis, but its efficacy in bacterial meningitis caused by penicillin- and cephalosporin-resistant pneumococcal organisms, Enterobacter species, and P. aeruginosa has not been established. A 2-week course of intravenous antimicrobial therapy is recommended for pneumococcal meningitis.
Table 26-1. Cerebrospinal Fluid (CSF) Analysis in Bacterial Meningitis | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 26-2. Antimicrobial Therapy of Bacterial Meningitis | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
published from 1988 to 1996 confirmed benefit for H. influenzae type b meningitis if dexamethasone was begun with or before intravenous antibiotics and suggested benefit for pneumococcal meningitis in children (10). In the European Dexamethasone in Adulthood Bacterial Meningitis Study of 301 adults with bacterial meningitis, 157 patients were randomized to receive dexamethasone and 144 patients were assigned to placebo 15 to 20 minutes before the first dose of an antimicrobial agent. There were 108 cases of pneumococcal meningitis. Within 14 days, five (9%) of 58 patients with pneumococcal meningitis died in the dexamethasone group and 13 (26%) of 50 patients in the placebo group died (2). In addition to reducing mortality, dexamethasone was associated with a reduction in the number of patients who had an unfavorable outcome. Present recommendations are to initiate dexamethasone therapy (10 mg) before or with the first dose of antibiotics and continue dexamethasone, regardless of the bacteria causing meningitis, in a dose of 10 mg every 6 hours for 4 days (23).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree