Normal Sleep and Sleep-Wake Disorders
 16.1 Normal Sleep
16.1 Normal Sleep
Sleep is one of the most significant of human behaviors, occupying roughly one third of human life. It is a universal behavior that has been demonstrated in every animal species studied, from insects to mammals. Sleep is a process the brain requires for proper functioning. Prolonged sleep deprivation leads to severe physical and cognitive impairment and, eventually, death. Sleep may appear to be a passive process but in fact can be associated with a high degree of brain activation. There are several distinct types of sleep that differ both qualitatively and quantitatively. Each type of sleep has unique characteristics, functional importance, and regulatory mechanisms. Selectively depriving a person of one particular type of sleep produces compensatory rebound when the individual is allowed to sleep ad lib. Sleep is particularly relevant to psychiatry since sleep disturbances can occur in virtually all psychiatric illnesses and are frequently part of the diagnostic criteria for specific disorders.
The ancient Greeks ascribed the need for sleep to the god Hypnos (sleep) and his son Morpheus, also a creature of the night, who brought dreams in human forms. Dreams have played an important role in psychoanalysis. Freud believed dreams to be the “royal road to the unconscious.” They have figured prominently in art and literature from ancient times to the present.
ELECTROPHYSIOLOGY OF SLEEP
Sleep is made up of two physiological states: non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. In NREM sleep, which is composed of stages 1 through 4, most physiological functions are markedly lower than in wakefulness. REM sleep is a qualitatively different kind of sleep, characterized by a high level of brain activity and physiological activity levels similar to those in wakefulness. About 90 minutes after sleep onset, NREM yields to the first REM episode of the night. This REM latency of 90 minutes is a consistent finding in normal adults; shortening of REM latency frequently occurs with such disorders as narcolepsy and depressive disorders.
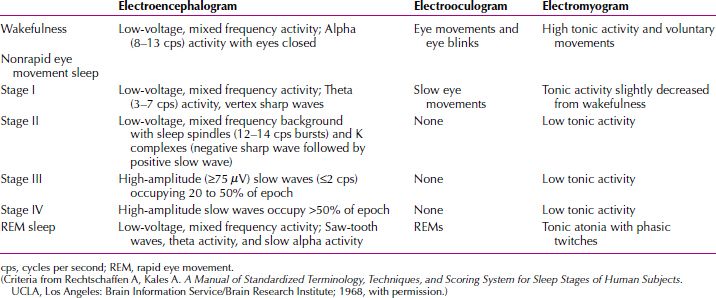
For clinical and research applications, sleep is typically scored in epochs of 30 seconds, with stages of sleep defined by the visual scoring of three parameters: electroencephalogram (EEG), electro-oculogram (EOG), and electromyogram (EMG) recorded beneath the chin. The EEG records the rapid conjugate eye movements that are the identifying feature of the sleep state (no or few rapid eye movements occur in NREM sleep); the EEG pattern consists of low-voltage, random, fast activity with sawtooth waves (Fig. 16.1-1); the EMG shows a marked reduction in muscle tone. The criteria defined by Allan Rechtschaffen and Anthony Kales in 1968 are accepted in clinical practice and for research around the world (Table 16.1-1).
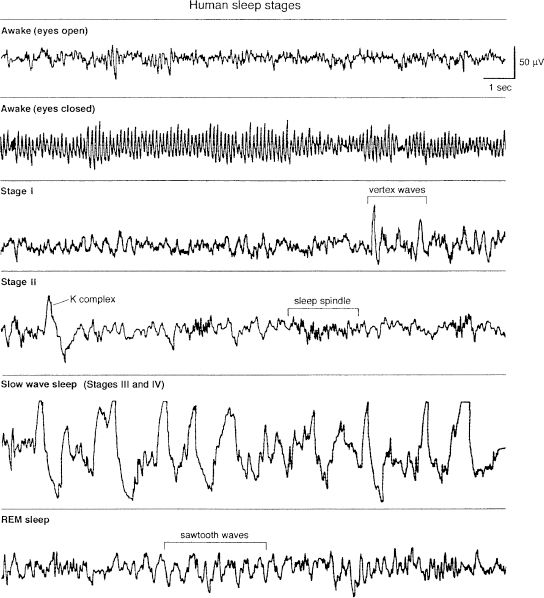
FIGURE 16.1-1
Electroencephalogram patterns for stages of human sleep and wakefulness. REM, rapid eye movement. (From Butkov N. Atlas of Clinical Polysomnography. Medford, OR: Synapse Media; 1996, with permission.)
 Table 16.1-1
Table 16.1-1
Stages of Sleep—Electrophysiological Criteria
In normal persons, NREM sleep is a peaceful state relative to waking. The pulse rate is typically slowed five to ten beats a minute below the level of restful waking and is very regular. Respiration is similarly affected, and blood pressure also tends to be low, with few minute-to-minute variations. The body musculature resting muscle potential is lower in REM sleep than in a waking state. Episodic, involuntary body movements are present in NREM sleep. There are few, if any, REMs and seldom do any penile erections occur in men. Blood flow through most tissues, including cerebral blood flow, is slightly reduced.
The deepest portions of NREM sleep—stages 3 and 4—are sometimes associated with unusual arousal characteristics. When persons are aroused 30 minutes to 1 hour after sleep onset—usually in slow-wave sleep—they are disoriented, and their thinking is disorganized. Brief arousals from slow-wave sleep are also associated with amnesia for events that occur during the arousal. The disorganization during arousal from stage 3 or stage 4 may result in specific problems, including enuresis, somnambulism, and stage 4 nightmares or night terrors.
Polygraphic measures during REM sleep show irregular patterns, sometimes close to aroused waking patterns. Otherwise, if researchers were unaware of the behavioral stage and happened to be recording a variety of physiological measures (aside from muscle tone) during REM periods, they undoubtedly would conclude that the person or animal they were studying was in an active waking state. Because of this observation, REM sleep has also been termed paradoxical sleep. Pulse, respiration, and blood pressure in humans are all high during REM sleep—much higher than during NREM sleep and often higher than during waking. Even more striking than the level or rate is the variability from minute to minute. Brain oxygen use increases during REM sleep. The ventilatory response to increased levels of carbon dioxide (CO2) is depressed during REM sleep, so that no increase in tidal volume occurs as the partial pressure of carbon dioxide (PCO2) increases. Thermoregulation is altered during REM sleep. In contrast to the homoeothermic condition of temperature regulation during wakefulness or NREM sleep, a poikilothermic condition (a state in which animal temperature varies with the changes in the temperature of the surrounding medium) prevails during REM sleep. Poikilothermia, which is characteristic of reptiles, results in a failure to respond to changes in ambient temperature with shivering or sweating, whichever is appropriate to maintaining body temperature. Almost every REM period in men is accompanied by a partial or full penile erection. This finding is clinically significant in evaluating the cause of impotence; the nocturnal penile tumescence study is one of the most commonly requested sleep laboratory tests. Another physiological change that occurs during REM sleep is the near-total paralysis of the skeletal (postural) muscles. Because of this motor inhibition, body movement is absent during REM sleep. Probably the most distinctive feature of REM sleep is dreaming. Persons awakened during REM sleep frequently (60 to 90 percent of the time) report that they had been dreaming. Dreams during REM sleep are typically abstract and surreal. Dreaming does occur during NREM sleep, but it is typically lucid and purposeful.
The cyclical nature of sleep is regular and reliable; a REM period occurs about every 90 to 100 minutes during the night (Fig. 16.1-2). The first REM period tends to be the shortest, usually lasting less than 10 minutes; later REM periods may last 15 to 40 minutes each. Most REM periods occur in the last third of the night, whereas most stage 4 sleep occurs in the first third of the night.
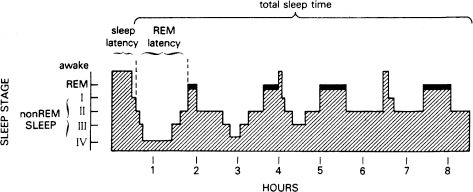
FIGURE 16.1-2
Sleep pattern in a young, healthy subject. REM, rapid eye movement. (From Gillian JC, Seifritz E, Zoltoltoski RK, Salin-Pascual RJ. Basic science of sleep. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 7th ed. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2000:199, with permission.)
These sleep patterns change over a person’s life span. In the neonatal period, REM sleep represents more than 50 percent of total sleep time, and the EEG pattern moves from the alert state directly to the REM state without going through stages 1 through 4. Newborns sleep about 16 hours a day, with brief periods of wakefulness. By 4 months of age, the pattern shifts so that the total percentage of REM sleep drops to less than 40 percent, and entry into sleep occurs with an initial period of NREM sleep. By young adulthood, the distribution of sleep stages is as follows:
NREM (75 percent)
Stage 1: 5 percent
Stage 2: 45 percent
Stage 3: 12 percent
Stage 4: 13 percent
REM (25 percent)
This distribution remains relatively constant into old age, although a reduction occurs in both slow-wave sleep and REM sleep in older persons.
SLEEP REGULATION
Most researchers think that there is not one simple sleep control center but a small number of interconnecting systems or centers that are located chiefly in the brainstem and that mutually activate and inhibit one another. Many studies also support the role of serotonin in sleep regulation. Prevention of serotonin synthesis or destruction of the dorsal raphe nucleus of the brainstem, which contains nearly all the brain’s serotonergic cell bodies, reduces sleep for a considerable time. Synthesis and release of serotonin by serotonergic neurons are influenced by the availability of amino acid precursors of this neurotransmitter, such as L-tryptophan. Ingestion of large amounts of L-tryptophan (1 to 15 g) reduces sleep latency and nocturnal awakenings. Conversely, L-tryptophan deficiency is associated with less time spent in REM sleep. Norepinephrine-containing neurons with cell bodies located in the locus ceruleus play an important role in controlling normal sleep patterns. Drugs and manipulations that increase the firing of these noradrenergic neurons markedly reduce REM sleep (REM-off neurons) and increase wakefulness. In humans with implanted electrodes (for the control of spasticity), electrical stimulation of the locus ceruleus profoundly disrupts all sleep parameters.
Brain acetylcholine is also involved in sleep, particularly in the production of REM sleep. In animal studies, the injection of cholinergic-muscarinic agonists into pontine reticular formation neurons (REM-on neurons) results in a shift from wakefulness to REM sleep. Disturbances in central cholinergic activity are associated with the sleep changes observed in major depressive disorder. Compared with healthy persons and nondepressed psychiatric controls, patients who are depressed have marked disruptions of REM sleep patterns. These disruptions include shortened REM latency (60 minutes or less), an increased percentage of REM sleep, and a shift in REM distribution from the last half to the first half of the night. Administration of a muscarinic agonist, such as arecoline, to depressed patients during the first or second NREM period results in a rapid onset of REM sleep. Depression can be associated with an underlying supersensitivity to acetylcholine. Drugs that reduce REM sleep, such as antidepressants, produce beneficial effects in depression. Indeed, about half the patients with major depressive disorder experience temporary improvement when they are deprived of sleep or when sleep is restricted. Conversely, reserpine (Serpasil), one of the few drugs that increase REM sleep, also produces depression. Patients with dementia of the Alzheimer’s type have sleep disturbances characterized by reduced REM and slow-wave sleep. The loss of cholinergic neurons in the basal forebrain has been implicated as the cause of these changes.
Melatonin secretion from the pineal gland is inhibited by bright light, so the lowest serum melatonin concentrations occur during the day. The suprachiasmatic nucleus of the hypothalamus may act as the anatomical site of a circadian pacemaker that regulates melatonin secretion and the entrainment of the brain to a 24-hour sleep-wake cycle. Evidence shows that dopamine has an alerting effect. Drugs that increase dopamine concentrations in the brain tend to produce arousal and wakefulness. In contrast, dopamine blockers, such as pimozide (Orap) and the phenothiazines, tend to increase sleep time. A hypothesized homeostatic drive to sleep, perhaps in the form of an endogenous substance—process S—may accumulate during wakefulness and act to induce sleep. Another compound—process C—may act as a regulator of body temperature and sleep duration.
FUNCTIONS OF SLEEP
The functions of sleep have been examined in a variety of ways. Most investigators conclude that sleep serves a restorative, homeostatic function and appears to be crucial for normal thermoregulation and energy conservation. As NREM sleep increases after exercise and starvation, this stage may be associated with satisfying metabolic needs.
Sleep Deprivation
Prolonged periods of sleep deprivation sometimes lead to ego disorganization, hallucinations, and delusions. Depriving persons of REM sleep by awakening them at the beginning of REM cycles increases the number of REM periods and the amount of REM sleep (rebound increase) when they are allowed to sleep without interruption. REM-deprived patients may exhibit irritability and lethargy. In studies with rats, sleep deprivation produces a syndrome that includes a debilitated appearance, skin lesions, increased food intake, weight loss, increased energy expenditure, decreased body temperature, and death. The neuroendocrine changes include increased plasma norepinephrine and decreased plasma thyroxine levels.
Sleep Requirements
Some persons are normally short sleepers who require fewer than 6 hours of sleep each night to function adequately. Long sleepers are those who sleep more than 9 hours each night to function adequately. Long sleepers have more REM periods and more rapid eye movements within each period (known as REM density) than short sleepers. These movements are sometimes considered a measure of the intensity of REM sleep and are related to the vividness of dreaming. Short sleepers are generally efficient, ambitious, socially adept, and content. Long sleepers tend to be mildly depressed, anxious, and socially withdrawn. Sleep needs increase with physical work, exercise, illness, pregnancy, general mental stress, and increased mental activity. REM periods increase after strong psychological stimuli, such as difficult learning situations and stress, and after the use of chemicals or drugs that decrease brain catecholamines.
Sleep-Wake Rhythm
Without external clues, the natural body clock follows a 25-hour cycle. The influence of external factors—such as the light-dark cycle, daily routines, meal periods, and other external synchronizers—entrain persons to the 24-hour clock. Sleep is also influenced by biological rhythms. Within a 24-hour period, adults sleep once, sometimes twice. This rhythm is not present at birth but develops over the first 2 years of life. Some women exhibit sleep pattern changes during the phases of the menstrual cycle. Naps taken at different times of the day differ greatly in their proportions of REM and NREM sleep. In a normal nighttime sleeper, a nap taken in the morning or at noon includes a great deal of REM sleep, whereas a nap taken in the afternoon or the early evening has much less REM sleep. A circadian cycle apparently affects the tendency to have REM sleep. Sleep patterns are not physiologically the same when persons sleep in the daytime or during the time when they are accustomed to being awake; the psychological and behavioral effects of sleep differ as well. In a world of industry and communications that often functions 24 hours a day, these interactions are becoming increasingly significant. Even in persons who work at night, interference with the various rhythms can produce problems. The best-known example is jet lag, in which, after flying east to west, persons try to convince their bodies to go to sleep at a time that is out of phase with some body cycles. Most persons adapt within a few days, but some require more time. Conditions in these persons’ bodies apparently involve long-term cycle disruption and interference.
REFERENCES
Barclay NL, Gregory AM. Quantitative genetic research on sleep: A review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013;17(1):29–40.
Benca RM, Cirelli C, Rattenborg NC, Tononi G. Basic science of sleep. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 8th ed. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2005:280.
Genderson MR, Rana BK, Panizzon MS, Grant MD, Toomey R, Jacobson KC, Xian H, Cronin-Golomb A, Franz CE, Kremen WS, Lyons MJ. Genetic and environmental influences on sleep quality in middle‐aged men: A twin study. J Sleep Res. 2013;22(5):519–526.
Gillin JC, Seifritz E, Zoltoski RK, Salin-Pascual R. Basic science of sleep. In: Sadock BJ, Sadock VA, eds. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 7th ed. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2000:199.
Jenni OG. How much sleep is “normal” in children and adolescents? Normal sleep duration in children and adolescents. JAMA Pediatr. 2013;167(1):91–92.
Potts KJ, Butterfield DT, Sims P, Henderson M, Shames CB. Cost savings associated with an education campaign on the diagnosis and management of sleep-disordered breathing: A retrospective, claims-based US study. Popul Health Manag. 2013;16(1):7–13.
Richardson GS. The human circadian system in normal and disordered sleep. J Clin Psychiatry. 2005;66(Suppl 9):3–9.
Rosipal R, Lewandowski A, Dorffner G. In search of objective components for sleep quality indexing in normal sleep. Biol Psychology. 2013;94(1):210–220.
Roth T. Characteristics and determinants of normal sleep. J Clin Psychol. 2004;65(Suppl 16):8–11.
Thomas SJ, Lichstein KL, Taylor DJ, Riedel BW, Bush AJ. Epidemiology of bedtime, arising time, and time in bed: Analysis of age, gender, and ethnicity. Behav Sleep Med. 2014;12(3):169–182.
Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50.
 16.2 Sleep-Wake Disorders
16.2 Sleep-Wake Disorders
Sleep is regulated by several basic mechanisms, and when these systems go awry, sleep disorders occur. Interest in sleep disorders was initially found among psychiatrists, psychologists, and neurologists. The past three decades have witnessed discoveries that make sleep medicine truly multidisciplinary. Research illustrating the medical consequences of sleep-disordered breathing attracted many pulmonary and internal medicine specialists to the field. Sleep-wake disorder–related endocrinology and circadian rhythm research has migrated from the laboratory bench to the bedside. Nonetheless, the seriousness of sleep disorders remains poorly recognized by the general public and the vast majority of clinical practitioners.
Sleep disorders are both dangerous and expensive to treat. Obstructive sleep apnea research verifies its contribution to hypertension, heart failure, and stroke. Investigations link many major industrial catastrophes to sleepiness. Sleepiness is a serious, potentially life-threatening condition that affects not only the sleepy individual but also his or her family, coworkers, and society in general. In fact, sleep-related motor vehicle accidents represent a major public safety concern, and some states have enacted criminal statues to deter sleepy driving. Sleep disorders’ direct cost per annum in the United States is estimated at $16 billion, with indirect costs ranging upward to more than $100 billion. Table 16.2-1 lists the terms used in this section to diagnose and describe sleep disorders.
 Table 16.2-1
Table 16.2-1
Common Polysomnographic Measures

SLEEP DISORDER CLASSIFICATION
DSM-5
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) of the American Psychiatric Association (APA) lists ten disorders or disorder groups as sleep-wake disorders. The DSM-5 classifies sleep disorders on the basis of clinical diagnostic criteria and presumed etiology. The disorders described in DSM-5 are only a fraction of the known sleep disorders; they provide a framework for clinical assessment. The sleep-wake disorders’ current classifications in accordance with the DSM-5 include the following:
1. Insomnia Disorder
2. Hypersomnolence Disorder
3. Narcolepsy
4. Breathing-Related Sleep Disorders:
a. Obstructive Sleep Apnea Hypopnea
b. Central Sleep Apnea
i. Idiopathic central sleep apnea
ii. Cheyne-Stokes breathing
iii. Central sleep apnea comorbid with opioid use
c. Sleep-Related Hypoventilation
5. Circadian Rhythm Sleep-Wake Disorders:
a. Delayed sleep phase type
b. Advanced sleep phase type
c. Irregular sleep-wake type
d. Non-24-hour sleep-wake type
e. Shift work type
f. Unspecified type
6. Parasomnias
7. Non-Rapid Eye Movement Sleep Arousal Disorders:
a. Sleepwalking type
b. Sleep terror type
8. Nightmare Disorder
9. Rapid Eye Movement Sleep Behavior Disorder
10. Restless Legs Syndrome
11. Substance/Medication-Induced Sleep Disorder
Other Classification Systems
ICSD-2. A different classification system of sleep-wake disorders is used by the American Sleep Disorders Association published in the second edition of International Classification of Sleep Disorders: Diagnostic and Coding Manual (ICSD-2). ICSD-2 provides a detailed and comprehensive classification system for sleep-wake disorders. Table 16.2-2 presents an outline of this classification.
 Table 16.2-2
Table 16.2-2
Outline of Sleep-Wake Disorders in the Second Edition of the International Classification of Sleep Disorders

ICD-10. The tenth revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) of the World Health Organization (WHO) includes many (but not all) of the ICSD-2 diagnostic classifications. In addition, their organizational schemas differ from DSM-5 and often lump multiple nosological entities into a single diagnostic classification. The subject of sleep disorders covers only those of nonorganic type in ICD-10. These disorders are classified as dyssomnias, psychogenic conditions “in which the predominant disturbances…[are] in the amount, quality, or timing of sleep” because of emotional causes, and parasomnias, “abnormal episodic events occurring during sleep.” The dyssomnias include insomnia, hypersomnia, and disorder of the sleep-wake schedule. The parasomnias in childhood are related to development; those in adulthood are psychogenic and include sleepwalking, sleep terrors, and nightmares. Sleep disorders of organic origin, nonpsychogenic disorders such as narcolepsy and cataplexy, and sleep apnea and episodic movement disorders are discussed under other categories.
The ICD-10 notes that sleep disorders are often symptoms of other disorders, but even when they are not, the specific sleep disorder should be diagnosed along with as many other relevant diagnoses as necessary to describe the “psychopathology and/or pathophysiology involved in a given case.” Table 16.2-3 presents the ICD-10 criteria for nonorganic sleep disorders.
 Table 16.2-3
Table 16.2-3
ICD-10 Diagnostic Criteria for Nonorganic Sleep Disorders

INSOMNIA DISORDER
Insomnia is defined as difficulty initiating or maintaining sleep. It is the most common sleep complaint and may be transient or persistent. Population surveys show a 1-year prevalence rate of 30 to 45 percent in adults.
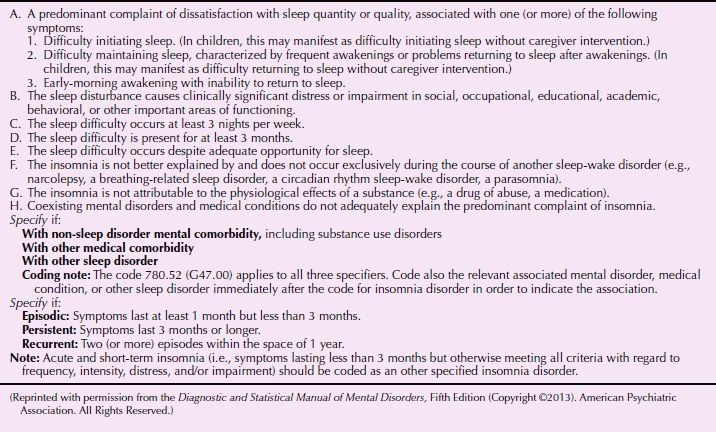
DSM-5 defines insomnia disorder as dissatisfaction with sleep quantity or quality associated with one or more of the following symptoms: difficulty in initiating sleep, difficulty in maintaining sleep with frequent awakenings or problems returning to sleep, and early morning awakening with inability to return to sleep (Table 16.2-4).
 Table 16.2-4
Table 16.2-4
DSM-5 Diagnostic Criteria for Insomnia Disorder
It is now recognized that insomnia can be an independent condition. In the past, practitioners were admonished to treat insomnia’s cause rather than the symptoms. There was an implicit notion that by doing so, the sleep problems would improve. Clinical experience suggested otherwise. Consequently, current therapeutics favor providing relief and managing symptoms. In the past it was argued that if insomnia was related to depression, treating the insomnia would mask the depression and thereby interfere with antidepressant treatment regimens. This does not appear to happen.
Descriptively, insomnia can be categorized in terms of how it affects sleep (e.g., sleep-onset insomnia, sleep-maintenance insomnia, or early-morning awakening). Insomnia can also be classified according to its duration (e.g., transient, short term, and long term). According to the Gallup Survey, approximately one third of the US population has several serious bouts of insomnia yearly; however, in 9 percent of the general population, insomnia is a chronic condition. Individuals with chronic insomnia have more than twice as many motor vehicle accidents as the general population, but only 5 percent of those with chronic insomnia see a health care provider to seek help for sleeplessness. Nonetheless, 40 percent or more of those individuals with chronic insomnia self-medicate with over-the-counter drugs, alcohol, or both.
A brief period of insomnia is most often associated with anxiety, either as a sequela to an anxious experience or in anticipation of an anxiety-provoking experience (e.g., an examination or an impending job interview). In some persons, transient insomnia of this kind may be related to grief, loss, or almost any life change or stress. The condition is not likely to be serious, although a psychotic episode or a severe depression sometimes begins with acute insomnia. Specific treatment for the condition is usually not required. When treatment with hypnotic medication is indicated, both the physician and the patient should be clear that the treatment is of short duration and that some symptoms, including a brief recurrence of the insomnia, may be expected when the medication is discontinued.
Persistent insomnia is composed of a fairly common group of conditions in which the problem is difficulty falling asleep or remaining asleep. This insomnia involves two sometimes separable, but often intertwined, problems: somatized tension and anxiety and a conditioned associative response. Patients often have no clear complaint other than insomnia. They may not experience anxiety per se but discharge the anxiety through physiological channels; they may complain chiefly of apprehensive feelings or ruminative thoughts that appear to keep them from falling asleep. Sometimes (but not always) a patient describes the condition’s exacerbation at times of stress at work or at home and its remission during vacations.
Sleep state misperception (also known as subjective insomnia) is characterized by a dissociation between the patient’s experience of sleeping and the objective polygraphic measures of sleep. The ultimate cause of this dissociation is not yet understood, although it appears to be a specific case of a general phenomenon seen in many areas of medicine. Sleep state misperception is diagnosed when a patient complains of difficulty initiating or maintaining sleep and no objective evidence of sleep disruption is found. For example, a patient sleeping in the laboratory reports taking more than an hour to fall asleep, awakening more than 30 times, and sleeping less than 2 hours the entire night. By contrast, the polysomnogram shows sleep onset occurring within 15 minutes, few awakenings, a 90 percent sleep efficiency, and total sleep time exceeding 7 hours. Sleep state misperception can occur in individuals who are apparently free from psychopathology or it can represent a somatic delusion or hypochondriasis. Some patients with sleep state misperception have obsessional features concerning somatic functions. Short-term sleep state misperception can occur during periods of stress, and some clinicians believe it can result from latent or ineffectively treated anxiety or depressive disorders. Cognitive relabeling, diffusing the worry about being unable to sleep, or both can help. Interestingly, anxiolytics can profoundly reduce the perception of sleeplessness without markedly changing sleep physiologically.
Psychophysiological insomnia typically presents as a primary complaint of difficulty in going to sleep. A patient may describe this as having gone on for years and usually denies that it is associated with stressful periods in his or her life. Objects associated with sleep (e.g., the bed, the bedroom) likewise become conditioned stimuli that evoke insomnia. Thus, psychophysiological insomnia is sometimes called conditioned insomnia. Psychophysiological insomnia often occurs in combination with other causes of insomnia, including episodes of stress and anxiety disorders, delayed sleep phase syndrome, and hypnotic drug use and withdrawal. In contrast to the insomnia in patients with psychiatric disorders, daytime adaptation is generally good. Work and relationships are satisfying; however, extreme tiredness can exist. Other features include (1) excessive worry about not being able to sleep; (2) trying too hard to sleep; (3) rumination, inability to clear one’s mind while trying to sleep; (4) increased muscle tension when attempting to sleep; (5) other somatic manifestations of anxiety; (6) being able to sleep better away from one’s own bedroom; and (7) being able to fall asleep when not trying (e.g., watching television). The sleep complaint becomes fixed over time. Interestingly, many patients with psychophysiological insomnia sleep well in the laboratory.
Ms. W, a 41-year-old divorced white woman, presented with a 2.5-year complaint of sleeplessness. She had some difficulty falling asleep (30 to 45 minutes sleep onset latency) and awakened every hour or two after sleep onset. These awakenings could last 15 minutes to several hours, and she estimated approximately 4.5 hours of sleep on an average night. She rarely took daytime naps notwithstanding feeling tired and edgy. She described her sleep problem as follows: “It seems like I never get into a deep sleep. I have never been a heavy sleeper, but now the slightest noise wakes me up. Sometimes I have a hard time getting my mind to shut down.” She viewed the bedroom as an unpleasant place of sleeplessness and stated, “I tried staying at a friend’s house where it is quiet, but then I couldn’t sleep because of the silence.”
At times, Ms. W would be unsure whether she was asleep or awake. She had a history of clock watching (to time her wakefulness) but stopped doing this when she realized it was contributing to the problem. Reportedly the insomnia is unrelated to seasonal changes, menstrual cycle, or time-zone translocation. Her basic sleep hygiene was good. Appetite and libido were unchanged. She denied mood disturbance, except that she was quite frustrated and concerned about sleeplessness and its effect on her work. Her work involved sitting at a microscope for 6 hours of a 9-hour working day and meticulously documenting her findings. Her final output had not suffered, but she now had to “double check” for accuracy.
She described herself as a worrier and a Type A personality. She did not know how to relax. For example, on vacation she continually worried about things that could go wrong. She could not even begin to unwind until she had arrived at the destination, checked in, and unpacked. Even then, she was unable to relax.
Medical history was unremarkable except for tonsillectomy (age 16 years), migraine headaches (current), and diet-controlled hypercholesterolemia. She took naproxen (Aleve) as needed for headache. She did not drink caffeinated beverages, smoke tobacco, or drink alcoholic beverages. She did not use recreational drugs.
The problem with insomnia began after relocation to a new city and place of employment. She attributed her insomnia to the noisy neighborhood in which she lived. She first sought treatment 18 months previously. Her family practice physician diagnosed depression and she was started on fluoxetine (Prozac), which made her “climb the walls.” Antihistamines were tried next with similar results. She was then switched to low-dose trazodone (Desyrel; for sleep) and developed nausea. After these medical interventions, she sought medical care elsewhere. Zolpidem (Ambien), 5 mg, was prescribed, but it made her feel drugged, and on discontinuation she had withdrawal effects. Another family practice physician diagnosed “nonspecific anxiety disorder” and began buspirone (BuSpar), an experience she described as “having an alien try to climb out of my skin.” Buspirone was discontinued. Paroxetine (Paxil) was tried for 8 weeks with no effect. Finally, a psychiatrist was consulted, who diagnosed adult attention-deficit disorder (without hyperactivity) and suggested treatment with methylphenidate (Ritalin). At this point, the patient was convinced that a stimulant would not help her insomnia and demanded referral to a sleep disorders center.
Ms. W’s symptoms fell into the broad category of insomnia, and the symptoms had begun after she had moved from one city to another. Environmental sleep disorder (noise) and adjustment sleep disorder (new job, city, and apartment) were likely initial diagnoses. However, a more chronic, endogenous problem had become operative. Ms. W was a “worrier” and meticulous, but she did not reach diagnostic criteria for personality or anxiety disorders. Dyssomnia associated with mood disorder should be considered in any patient with sleep maintenance problems and early-morning awakening insomnia. However, this patient did not have other significant signs of depression. Unfortunately, many patients are misdiagnosed with depression or “masked depression” on the sole basis of an insomnia complaint and unsuccessfully treated with antidepressant medication. Ms. W’s job demanded long hours with focused concentration. Her job performance had been superior for many years notwithstanding insomnia. Thus, a diagnosis of attention-deficit disorder was unlikely. Idiopathic insomnia implies a childhood complaint, which Ms. W denied.
The likely working diagnosis was psychophysiological insomnia (PPI). There may have been some sleep state misperception (she was sometimes unclear on whether she was awake or asleep), but this could not adequately account for the constellation of symptoms. An initial treatment plan should include further documentation of the sleep pattern using a sleep log. Behavioral treatments would likely benefit this patient. Medications with sedative effects are sometimes useful during the initial treatment of PPI. However, thus far in this patient they had done more harm than good. She would likely be a challenging patient to treat. (Courtesy of Max Hirshkowitz, Ph.D., Rhoda G. Seplowitz-Hafkin, M.D., and Amir Sharafkhaneh, M.D., Ph.D.)
Idiopathic insomnia typically starts early in life, sometimes at birth, and continues throughout life. As the name implies, its cause is unknown; suspected causes include neurochemical imbalance in brainstem reticular formation, impaired regulation of brainstem sleep generators (e.g., raphe nuclei, locus ceruleus), or basal forebrain dysfunction. Treatment is difficult, but improved sleep hygiene, relaxation therapy, and judicious use of hypnotic medicines are reportedly helpful.
Primary insomnia is diagnosed when the chief complaint is nonrestorative sleep or difficulty in initiating or maintaining sleep, and the complaint continues for at least a month (according to ICD-10, the disturbance must occur at least three times a week for a month). The term primary indicates that the insomnia is independent of any known physical or mental condition. Primary insomnia is often characterized both by difficulty falling asleep and by repeated awakening. Increased nighttime physiological or psychological arousal and negative conditioning for sleep are frequently evident. Patients with primary insomnia are generally preoccupied with getting enough sleep. The more they try to sleep, the greater the sense of frustration and distress and the more elusive sleep becomes.
Treating Insomnia
Pharmacological Treatment. Primary insomnia is commonly treated with benzodiazepines, zolpidem, eszopiclone (Lunesta), zaleplon (Sonata), and other hypnotics. Hypnotic drugs should be used with care. In general, sleep medications should not be prescribed for more than 2 weeks because tolerance and withdrawal may result. For many years, benzodiazepines were the most commonly prescribed sedative–hypnotic medications for treating insomnia. Benzodiazepine-receptor agonists represent the current standard for sedative–hypnotic medications used to treat insomnia. Long-acting sleep medications (e.g., flurazepam [Dalmane], quazepam [Doral]) are best for middle-of-the-night insomnia; short-acting drugs (e.g., zolpidem, triazolam [Halcion]) are useful for persons who have difficulty falling asleep. The melatonin-receptor agonist ramelteon (Rozerem) has also been approved for treating sleep-onset insomnia. Sedating antidepressants, such as trazodone, are also frequently prescribed as sleep aids.
A variety of over-the-counter (OTC) sleep aids are also available. Nonprescription formulas include sedating antihistamines, protein precursors, and other substances. L-Tryptophan was popular and readily available at health food stores until an outbreak of eosinophilia led to its being pulled off the shelves. Melatonin is a leader among self-administered food additives believed by some to alleviate sleeplessness. Melatonin is an endogenous hormone produced by the pineal gland, which is linked to the regulation of sleep. Administration of exogenous melatonin has yielded mixed results, however, in clinical research.
Prescription medicines are rigorously tested in clinical trials; therefore, they hold an advantage over the virtually untested OTCs. To attain U.S. Food and Drug Administration (FDA) approval as a hypnotic, a medication must be safe and effective. Most hypnotic medications are approved for short-term, not long-term, use. Exceptions include zolpidem modified release, eszopiclone, and ramelteon, all of which are approved for long-term therapy. When properly used, hypnotics can provide immediate and adequate relief from sleeplessness. Insomnia, however, usually returns on discontinuation of dosing.
Cognitive-Behavioral Therapy
Cognitive-behavioral therapy (CBT) as a treatment modality uses a combination of behavioral and cognitive techniques to overcome dysfunctional sleep behaviors, misperceptions, and distorted, disruptive thoughts about sleep. Behavioral techniques include universal sleep hygiene, stimulus control therapy, sleep restriction therapy, relaxation therapies, and biofeedback.
Studies repeatedly show significant, sustained improvement in sleep symptoms, including number and duration of awakenings and sleep latency from CBT. Short-term benefits are similar to that of medication, but CBT tends to have lasting benefits even 36 months after treatment. With cessation of the medication, insomnia frequently returns and is sometimes accompanied by rebound insomnia. CBT has not been shown to produce any adverse effects. There are no established “best practice” guidelines for length or quantity of sessions.
CBT, however, is not without limitations. Most data do not compare the efficacy of the individual components of CBT. However, sleep hygiene education alone produces an insignificant effect on sleep. In addition, there are no studies demonstrating evidence for improved efficacy with the combination of the aforementioned components or what cognitive therapy adds to the behavioral component. Intuitively, it would seem that the multicomponent approach addresses many of the variables contributing to insomnia.
The effects of CBT take longer to emerge than effects of medications. Usually when patients finally come for treatment of their insomnia, they are desperate. This makes it difficult to convince them to try a therapy that may take several weeks before it will provide relief. Furthermore, patients do not assume a passive role in this type of therapy; they must be active participants. Many individuals not only want a “quick fix,” but they also want to undergo a procedure or have something administered rather than be involved in the therapeutic process. For CBT to be effective, patients must commit to come to multiple sessions and also be open to the idea that modifying thoughts and behaviors about sleep can improve the symptoms of insomnia. The “quick fix” model is more familiar to primary care providers, whereas psychiatrists are used to the delayed response of antidepressants and other psychotropics. Therefore, psychiatrists may be more amenable to recommending CBT. Another barrier for physicians using CBT in clinical practice is that providing CBT for insomnia requires a greater time commitment than prescribing a sleep aid.
Although firmly focused on cognitive and behavioral issues, it helps to extend CBT just slightly into the psychodynamic sphere. For some patients with long-standing difficulty sleeping, being an insomniac becomes an important part of their identity. There may be primary or secondary gain to such identification. It is the negative emotional response (i.e., anger at the inability to control one’s sleep, feeling like a failure because one cannot sleep) to insomnia that contributes to its chronicity. In general, these individuals tend to internalize rather than express emotion, feel a heightened need for control, experience interpersonal difficulties, and have significant discontent with past events. For this subset of people, if the emotional response is not addressed, there is more likely to be a limited response to CBT or a relapse of insomnia over time. The clinician who is attuned to a patient’s tendency to view something as a failure rather than a challenge will be better able to intercept barriers to treatment.
Universal Sleep Hygiene. A common finding is that a patient’s lifestyle leads to sleep disturbance. This is usually phrased as inadequate sleep hygiene, referring to a problem in following generally accepted practices to aid sleep. These include, for instance, keeping regular hours of bedtime and arousal, avoiding excessive caffeine, not eating heavy meals before bedtime, and getting adequate exercise. Many behaviors can interfere with sleep and may do so by increasing nervous system arousal near bedtime or by altering circadian rhythms.
The focus of universal sleep hygiene is on modifiable environmental and lifestyle components that may interfere with sleep, as well as behaviors that may improve sleep. Treatment should focus on one to three problem areas at a time. Especially because some of these behaviors are difficult to change, only one or two items that are collaboratively chosen by the patient and clinician should be addressed. This gives the patient the best chance at a successful intervention. Overwhelming the patient with too many lifestyle changes or a complex regimen seldom succeeds. Some general “dos and don’ts” are instructive. Sleep-enhancing directives are enumerated in Table 16.2-5. Often a few simple alterations in a patient’s habits or sleep environment can be effective. The clinician, however, needs to spend time reviewing both the patient’s routine and its irregularity. In some respects, the essence of insomnia is its variability. The day-to-day changes in behavior and the changing severity of sleeplessness can obscure the factors responsible for the problem. A carefully explained program of sleep hygiene, with follow-up, represents a fairly inexpensive but effective intervention. Furthermore, improving sleep habits can enhance sleep even when the major cause of insomnia is physical.
 Table 16.2-5
Table 16.2-5
Dos and Don’ts for Good Sleep Hygiene
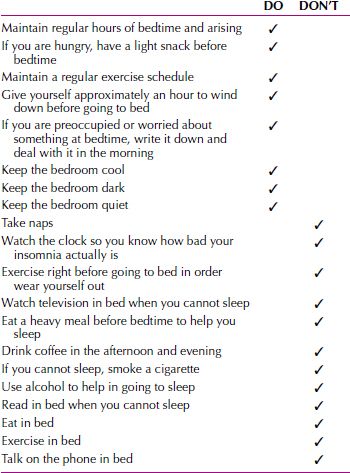
Stimulus Control Therapy. Stimulus control therapy is a deconditioning paradigm developed by Richard Bootzin and colleagues at the University of Arizona. This treatment aims to break the cycle of problems commonly associated with difficulty initiating sleep. By attempting to undo conditioning that undermines sleep, stimulus control therapy helps reduce both primary and reactive factors involved in insomnia. The rules attempt to enhance stimulus cues for sleeping and diminish associations with sleeplessness. The instructions are simple; however, they must be followed consistently. The first rule is, go to bed only when sleepy to maximize success. Second, use the bed only for sleeping. Do not watch television in bed, do not read, do not eat, and do not talk on the telephone while in bed. Third, do not lie in bed and become frustrated if unable to sleep. After a few minutes (do not watch the clock), get up, go to another room, and do something nonarousing until sleepiness returns. The goal is to associate the bed with rapid sleep onset. Rule three should be repeated as often as needed. The fourth and final instruction attempts to enhance the mechanisms underlying the circadian and sleep-wake cycles—that is, awaken at the same time every morning (regardless of bedtime, total sleep time, or day of week) and totally avoid napping. Stimulus control therapy does work; however, results might not be seen during the first few weeks or month. If continually practiced, the bouts of insomnia lessen in both frequency and severity.
Sleep Restriction Therapy. Sleep restriction therapy is a strategy designed to increase sleep efficiency by decreasing the amount of time spent awake while lying in bed. Developed by Arthur Spielman, this therapy specifically targets those patients who lie awake in bed unable to sleep. Restricting time in bed can help to consolidate sleep. If the patient reports sleeping only 5 hours of a scheduled 8-hour time in bed, reduce the time in bed. It is advised, however, not to reduce bedtime to less than 4 hours per night and to warn the patient about the hazards of daytime sleepiness. Sleep at other times during the day must be avoided, except in the elderly, who may take a 30-minute nap. The clinician then monitors sleep efficiency (time asleep as a percentage of the time in bed). When sleep efficiency reaches 85 percent (averaged over five nights), time in bed is increased by 15 minutes. Sleep restriction therapy produces a gradual and steady decline in nocturnal wakefulness.
Relaxation Therapy and Biofeedback. The most important aspects of relaxation therapy are that it be performed properly. Self-hypnosis, progressive relaxation, guided imagery, and deep breathing exercises are all effective if they produce relaxation. The goal is to find the optimal technique for each patient, but not all patients need help in relaxing. Progressive muscle relaxation is especially useful for patients who experience muscle tension. The patients should purposefully tense (5 to 6 seconds) and then relax (20 to 30 seconds) muscle groups, beginning at the head and ending at the feet. The patient should appreciate the difference between tension and relaxation. Guided imagery has the patient visualize a pleasant, restful scene, engaging all of his or her senses. Breathing exercises are practiced for at least 20 minutes per day for 2 weeks. Once mastered, the technique should be used once at bedtime for 30 minutes. If it does not work, the patient should try again another night. It is important that the technique not become associated with failure to fall asleep.
The patient is instructed to perform abdominal breathing as follows. The patient must become comfortable with each step before moving on to the next:
First, in the supine position, the patient should breathe normally through his or her mouth or nose, whichever is more comfortable, and attend to his or her breathing pattern.
Second, while maintaining that rhythm, the patient should begin to breathe more with his or her abdomen and less with his or her chest.
Third, the patient should pause for a half second after each breath cycle (in and out) and evaluate the breath. How did it feel? Was it smooth? Eventually each breath will become uniform and smooth.
Fourth, the patient should find a place where he or she can best feel the air move in and out. Concentrate on that spot and on the air moving in and out.
Fifth, the patient should visualize intrusive thoughts as floating away; if there are too many thoughts, stop practicing and try again later.
Biofeedback provides stimulus cues for physiological markers of relaxation and can increase self-awareness. A machine is used to measure muscle tension in the forehead or finger temperature. Finger temperature rises when a person becomes more relaxed. Patients require careful and adequate training; simply giving them an instruction tape is not especially helpful. Techniques are ideally mastered during the day for several weeks before application to the sleep problem; this is best achieved outside of the bed. By the time the techniques are applied in bed, the skill should be automatic. Relaxation techniques readily lend themselves to being combined with sleep hygiene and stimulus control therapies. Sometimes, they make for good distractions from thinking about the inability to sleep. The ruminations fuel the insomnia, and if the ruminator can be distracted, then the person may sleep better.
Cognitive Training.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








