
Clinical Controversies: Cavernous Malformations
Objectives: After reading this chapter, the reader should be able to select patients appropriately for conservative management, and describe all facets of medical management and clinical surveillance.
Accreditation: The AANS* is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to sponsor continuing medical education for physicians.
Credit: The AANS designates this educational activity for a maximum of 15 credits in Category 1 credit toward the AMA Physician’s Recognition Award. Each physician should claim only those hours of credit that he/she spent in the educational activity.
The Home Study Examination is online on the AANS Web site at: http://www.aans.org/education/books/controversy.asp
* The acronym AANS refers to both the American Association of Neurological Surgeons and the American Association of Neurosurgeons.
Cerebral cavernous malformations (CCMs) are known to be associated with intracerebral hemorrhage.1–4 They are commonly recognized on computerized tomography (CT) and magnetic resonance imaging (MRI) scans in both asymptomatic and symptomatic patients.5 The diagnosis of CCMs can often be made on an MRI scan based on the characteristic morphology of the subacute and chronic blood products. An atypical appearance of a CCM in the setting of a recent hemorrhage requires follow-up imaging to confirm the diagnosis.3,5
At pathological examination CCMs are rounded or lobulated collections of dilated single-cell-layer endothelium- lined vascular channels.6–10 Calcification and thickened collagen can be observed in association with some thrombosis.11,12 By definition, there is no smooth muscle and no elastin in these vascular channels. There is no intervening neural tissue, and gliosis and hemosiderin staining are common at the periphery of these lesions. Small arteries and veins enter and exit the lesion at the periphery. In biological terms, it is likely that the mechanisms of origin and growth are different for CCMs than other vascular anomalies. Efforts at characterizing the particular vascular growth factors that may underlie these differences will help provide an understanding of the biological differences.
 Epidemiology
Epidemiology
The prevalence of CCMs in the general population is not known2,13–22; however, the frequency of occurrence has been reported to be 0.02 to 0.5% based on autopsy studies.2,19,23 Detection rates of 0.39 to 0.9% are based on a retrospective review of MRI scans.3,5,24,25 Cerebral cavernous malformations do not appear to be more prevalent in females. Some have suggested that males under the age of 30 have a higher rate of detection, while the rate of detection is higher in women aged between 30 to 60 years. At 60??years, the frequency is similar for both men and women.3,26 In children, a bimodal age presentation has been described, with increased detection at age 3 and 11 years.21,27 Late presentation in elderly individuals is rare. It is likely that lesions invariably become symptomatic in life or not at all, or that aging may be associated with regression of CCM.
 Anatomical and Biological Characteristics
Anatomical and Biological Characteristics
These lesions present in a broad spectrum of size from several millimeters to 4 to 5 cm in diameter. The frequency of occurrence appears to correspond with the volume of various central nervous system (CNS) compartments, with 80% found supratentorially and 20% infratentorially (Table 21-1). Cerebral cavernous malformations can occur with other vascular anomalies. Cavernous malformations are commonly juxtaposed to deep venous malformations. This association has likely confounded the interpretation of the natural history of venous malformations. In a retrospective review, patients with CCMs associated with vascular malformations were more likely to be female patients who suffered symptomatic hemorrhage with lesions in the posterior fossa. They were also less likely to present with seizures or to have familial histories when compared with patients with CCMs alone.28 As documented in imaging studies, CCMs enlarge over time with de novo appearance of new lesions by MRI criteria.8,29–31 Precursor lesions such as capillary telangiectasias have been postulated, but origins remain obscure. It has been speculated that growth factors such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) are involved in the growth of these lesions32; their presence has been demonstrated by the immunostaining of surgical specimens. Ultrastructural studies suggest that abnormalities of the blood–brain barrier are important in the pathophysiology of cavernous malformations.33,34
Cerebral cavernous malformations occur in two forms: sporadic, generally producing a single, isolated lesion, and the autosomal dominant, frequently producing multiple lesions.12,31,35,36 The inherited form accounts for up to 50% of all cases of cavernous malformation. Familial cavernous malformation is genetically heterogenous, with at least three disease-causing loci. So far, CCM loci have been assigned to chromosomes 7q (CCM1), 7p (CCM2), and 3q (CCM3) and have been identified in 40%, 20%, and 40%, respectively, of families with CCM. Loss-of-function mutations have been identified in CCM1/KRIT1, the sole CCM gene identified to date.37–41 The loss of CCM1 leads to primary vascular defects and disrupts the molecular pathway regulating arterial identity.42–44 The gene responsible for CCM2 was recently discovered and named malcavernin or MGC4607.37,45 The gene(s) responsible for CCM3 still await identification.
Table 21-1 Anatomical Locations of Cavernous Malformations
Compartments | Most Common Lovsyiond | Number of Cases(%) |
|---|---|---|
Supratentorial | Temporal lobes | 80% |
Frontal and parietal lobes | ||
Occipital lobes | ||
Infratentorial | Pons cerebellum | 20% |
Table 21-2 Clinical Presentations of Cerebral Cavernous Malformations
Clinical Symptoms | No. of Cases (%) |
|---|---|
Seizures | 60 |
Focal Deficits | 40 |
Headache | 30 |
Asymptomatic | 15 |
 Clinical Presentations
Clinical Presentations
A cerebral cavernous malformation presents clinically as a seizure (60%), focal deficit (40%), or headache (30%), or without symptoms (15%) (Table 21-2). Seizures in CCM are related to brain irritation, neural compression, and local hemorrhages with exposure of the local brain parenchyma to blood products, particularly iron and later local gliotic reaction.8,26 The possible pathophysiological mechanisms of epileptogenesis include neuronal cell loss, glial proliferation and abnormal glial physiology, altered neurotransmitter levels, free radical formation, and aberrant second messenger physiology.46 In CCMs, temporal lobe location, heavy calcification, and extensive hemosiderin deposition are more likely to have epileptic presentation rather than gross hemorrhage.47,48
With the more liberal use of magnetic resonance imaging, the incidental or asymptomatic category will come to represent a larger percentage of the lesions identified. Given that one of the defining MRI characteristics is evidence of hemosiderin, it is important to emphasize that clinical hemorrhage associated with these lesions should be accompanied by frank hematoma and clear clinical symptoms.
 Diagnostic Evaluation
Diagnostic Evaluation
Definition by imaging studies has become most common in the era of magnetic resonance imaging. Cerebral cavernous malformations are rounded or lobulated lesions with a mixed signal core surrounded by low signal rim. Susceptibility sequences and high-resolution blood oxygenation level–dependent venography detect more lesions than conventional MRI sequences (Fig. 21-1).49–51 No definitive feeding artery or a draining vein can be identified by MR angiography or conventional contrast angiography. The clinician rarely has all of this information available for the classification of a particular lesion. This leads to difficulty in the evaluation of a suspected CCM (Fig. 21-2). It is not possible based on imaging studies alone to exclude a small thrombosed arteriovenous malformation; some clinicians therefore prefer to use a less-specific term of cryptic vascular malformation. The administration of gadolinium contrast is unnecessary for the diagnosis but may help identify venous malformations that frequently coexist with these lesions.49–51
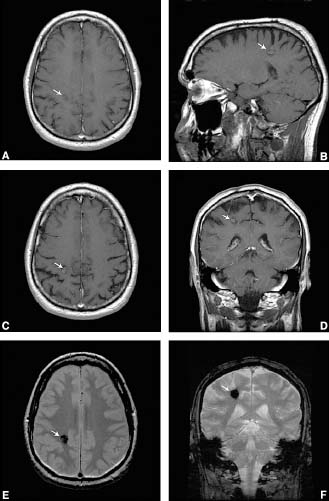
FIGURE 21-1 This 54-year-old man presented with a history of chronic daily headaches and two brief episodes of tingling involving his left hand. An abnormality seen on a head CT “offered” to him during a cardiac imaging session showed an abnormality, prompting a visit to the neurologist. His neurological examination was nonfocal. (A, B) Pregadolinium T1-weighted images demonstrate intrinsic T1 shortening with a surrounding rim of hypointensity. (C, D) Postgadolinium images demonstrate minimal enhancement. (E) This T2- weighted image has a prominent low signal caused by hemosiderin. (F) The multi-planar gradient (MPGR) sequences clearly show focal ovoid area of susceptibility in the right frontoparietal region right adjacent to the central sulcus.



