Upper-airway Resistance Syndrome
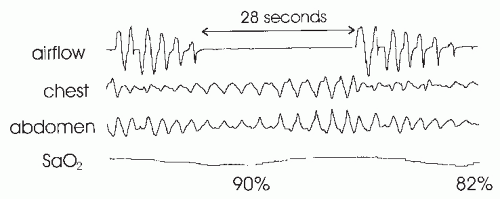
As noted earlier, Guilleminault and coworkers identified a group of patients who exhibited subjective and objective (Multiple Sleep Latency Test [MSLT]) daytime sleepiness but did not have an AHI of >5 per hour (thermal devices measured airflow). The group was defined by having an RAI of >10 per hour using esophageal pressure monitoring (
12). The events were not associated with desaturation or a change in thermal device-detected airflow. The symptom of sleepiness responded to CPAP treatment. The mean arousal index of the group was 33 per hour (range
16-52), and the mean maximally negative esophageal pressure nadir was -37 cm H
2O. There has been controversy as to whether the upper-airway resistance syndrome is a distinct entity or simply a milder form of OSAHS (
29,
30). Individuals without daytime sleepiness may have a RERA index of >10 per hour, although the mean RERA for a group of persons without symptoms is usually <10 per hour (
31). Of note, the mean total arousal index in a group of normal persons using AASM criteria was 21 per hour in one study (
32). The 95% confidence limit of normal for the arousal index was very wide due to high arousal rates in older patients. It is possible that respiratory arousals cause more potent sleep disruption than “spontaneous arousals.” However, this has never been experimentally addressed. In any case, there is some overlap in the arousal index between groups of normal subjects and patients with upper-airway resistance syndrome/mild OSAHS. If one uses nasal pressure monitoring for airflow and a definition of hypopnea that utilizes arousal, as well as a drop in the Sao
2, most “upper-airway resistance” patients will have an AHI of >5 per hour and, hence, be classified as having the OSAHS. Alternatively, an RDI = AHI + RERA index will be >5 per hour if hypopneas are required to be associated with a 4% or greater desaturation.
Obesity Hypoventilation Syndrome
Most patients with the OSAHS do not have daytime hypoventilation. Obese patients with daytime hypoventilation, not secondary to lung disease, are said to have the obesity hypoventilation syndrome (OHS). These patients were previously referred to as “Pickwickian patients” (
1). Patients with the OHS are a heterogeneous group. The etiology includes upper-airway obstruction, decreased respiratory system compliance from obesity, and intrinsic or acquired abnormalities in ventilatory drive. Most OHS patients will have a high AHI and severe arterial oxygen desaturation (
33,
34 and
35). A few will exhibit worsening hypoventilation and severe arterial oxygen desaturation during sleep, without many discrete apneas or hypopneas. A recent study characterized the patients on the basis of their response to treatment (
35). Some OHS patients could be adequately treated with CPAP alone. Opening the upper airway with CPAP during sleep restored adequate oxygenation. Others still had hypoventilation despite the absence of apnea or hypopnea. Some patients with persistent airflow limitation responded to higher levels of CPAP (decreasing the upper-airway resistance). Presumably, they could not compensate for a high upperairway resistance even if apnea and hypopnea were not present. Another group of patients required either nasal bilevel pressure-support ventilation or mechanical ventilation with or without oxygen. This group was felt likely to have abnormal ventilatory drive or very decreased respiratory compliance due to massive obesity. Despite the fact that the term OHS describes a very diverse group, a better terminology for the group of patients is not currently available. The sleep hypoventilation syndrome is an alternate term used by a task force of the AASM to include all forms of abnormal sleep-induced hypoventilation (
22).
OHS patients may present with acute respiratory failure (
36,
37). The treatment of choice is positive airway pressure (usually bilevel positive airway pressure and oxygen). Very severe patients may require temporary endotracheal intubation and mechanical ventilation. For stable chronic OHS patients, treatment with positive airway pressure may reduce the daytime PCO
2 as well as apnea and hypopnea and nocturnal desaturation (
33,
34 and
35). Berthon-Jones and Sullivan (
38) showed that chronic CPAP treatment of OSA patients with daytime hypoventilation resulted in a leftward shift in the ventilatory response to CO
2 (ventilation plotted vs. PCO
2) during the day without a change in slope. The PCO
2 set point is lowered, and there is higher ventilation at any given PCO
2. Medroxyprogesterone, a respiratory stimulant, may improve the daytime PCO
2 but does not reduce the AHI (
39).
Pathogenesis of Upper-airway Obstruction
A detailed discussion of the pathogenesis of upper-airway obstruction is beyond the scope of this chapter. The reader is referred to several excellent reviews on this topic (
45,
46). Multiple factors determine upper-airway patency during sleep (
Table 18-2). Different factors may be more or less important in a given individual. Patients with the OSAHS tend to have small upper airways either secondary to bony or soft-tissue alterations (
47). In general, a short and posteriorly placed mandible, a long dependent palate, a large tongue, nasal obstruction, and thick lateral pharyngeal walls all predispose to upper-airway collapse during sleep. Patients with OSAHS tend to have a different shape of the upper airway with the narrowest dimension laterally versus anterior-posterior in normal persons (
47,
48). Dynamic
imaging of the upper airway during wakefulness shows the smallest diameter at end expiration. Studies of the upper airway during general anesthesia (passive properties) have shown the upper airway of OSAHS patients to be narrower and more collapsible (
49). When the Starling resistor model is applied to the upper airway, one can define a critical closing pressure (
Pcrit) during sleep such that lower intraluminal pressures are associated with airway closure (
50). In normal persons,
Pcrit is negative, whereas in OSAHS patients, it is positive. That is, a positive intraluminal pressure is required to keep the airway open during sleep.
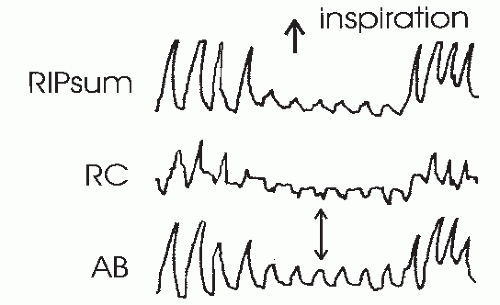
During wakefulness, upper-airway muscle activity maintains an open upper airway even if the airway is anatomically narrow. Some upper-airway muscles, such as the genioglossus (tongue protruder) and palatoglossus, show increases of activity with inspiration (phasic activity), while others such as the tensor veli palatini (a muscle of the palate) show tonic (constant) activity (
51). At the onset of NREM sleep, the activity of upper-airway muscles decreases (
51,
52 and
53) and upper-airway resistance increases (
54) (
Fig. 18-7). With stable sleep, the activity of the genioglossus may actually return to waking or higher than wakefulness levels. While chemostimulation from hypoxia and hypercapnia stimulates respiratory muscle activity during sleep, simultaneous increases in genioglossus activity appears to be related, in large part, to stimulation of upperairway mechanoreceptors by negative pressure (
55,
56). Upper-airway reflexes triggered by negative pressure also help maintain upper-airway patency during wakefulness. The sudden application of negative pressure elicits a reflex increase in the genioglossus (
57) and palatal muscle activity (
58). This reflex is diminished during sleep (
58,
59 and
60).
Patients with OSAHS tend to have higher than normal basal genioglossus activity (
61,
62) and a greater genioglossus response to negative airway pressure (
63). The higher activity and response to negative pressure are believed to be a compensation for an intrinsically narrowed airway. In contrast, the response of the palate muscles to negative pressure may be impaired in OSAHS patients (
64). Despite evidence for higher upper-airway muscle activity during wakefulness, the upper airways of OSAHS patients are still more collapsible than those of normal persons (
65). At sleep onset, some studies have suggested that OSAHS patients have a greater than normal fall in upperairway muscle activity (
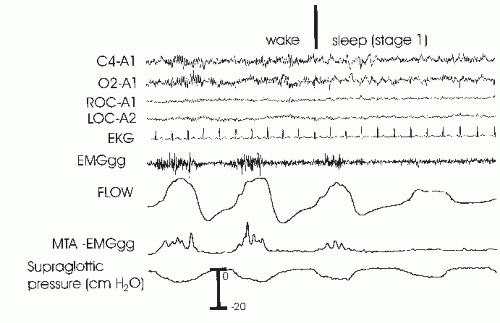
66). In any case, the upper-airway activity is not sufficient to maintain an open upper airway. In
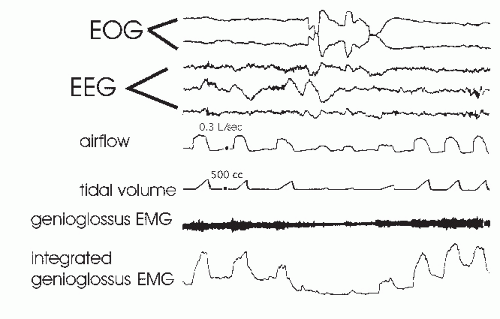
Figure 18-3, a fall in genioglossus activity as the patient returns to sleep is associated with an obstructive apnea. Posture also has important effects on airway patency (
67,
68), and some patients with OSAHS have apnea or hypopnea only in the supine position (postural OSAHS).
During upper-airway obstruction, phasic genioglossus muscle activity increases proportionally to esophageal pressure deflections (ventilatory drive) (see
Fig. 18-3) (
4,
69). At apnea termination, both genioglossus and palatal muscle activities are preferentially augmented (
4,
69,
70)
and the upper airway opens. While it was once assumed that increasing genioglossus activity during obstructive apnea or hypopnea was driven by hypoxia and hypercapnia, a study of the effect of upper-airway local anesthesia suggests that mechanoreceptor stimulation (from increasingly negative pressure below the site of airway closure) is responsible for a large proportion of the augmentation (
69). Traditionally a concept of a balance between negative inspiratory pressure tending to collapse the airway and upper-airway muscle dilating forces was assumed to determine the state of the airway. However, more recently the concept of passive collapse at sleep onset has gained favor. In fact, at sleep onset, the ventilatory drive decreases and supraglottic pressure actually may initially decrease (less negative) in some patients (although resistance increases). Upper-airway closure has also been documented during central apnea where there is no inspiration or negative collapsing forces (
71). Therefore, suction pressure during obstruction may help keep the airway closed but is not necessary for the onset of airway occlusion.
Upper-airway volume also has a dependence on lung volume, with decreasing airway size as lung volume decreases (
72,
73). The lung volume dependence may be greater in patients with OSAHS (
72). The lung volume dependence of the upper airway may be mediated via passive distending forces due to a downward tension on upper-airway structures during inspiration (tracheal tug) (
74). Another way of thinking of the tracheal tug is a decrease in extramural pressure surrounding the airway (
46). Any fall in end-expiratory volume or tidal volume would then reduce upper-airway size. Functional residual capacity (FRC) is known to decrease during sleep (
75), and this would tend to predispose to airway closure. Morrell and coworkers (
76) demonstrated a progressive fall in end-expiratory retropalatal cross-sectional area as well as end-expiratory lung volume in the breaths leading up to obstructive apnea.
Ventilatory instability generated by arousal and hyperventilation postapnea may also predispose to subsequent upper-airway closure and help perpetuate repetitive cycles of respiration and apnea. If the patient falls asleep rapidly after arousal, the arterial PCO
2 may be near or below the apneic threshold, the level of PCO
2 below which ventilation is no longer triggered during sleep
(77). If the PCO
2 falls below the apneic threshold, a central apnea may occur, followed by an obstructive apnea when ventilatory drive returns (mixed apnea) (
78). Alternatively, a hypopnea or obstructive apnea may occur as ventilatory drive and upper-airway muscle activity fall (
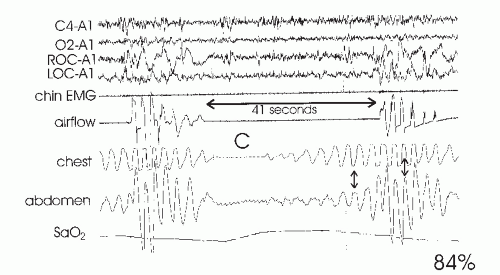
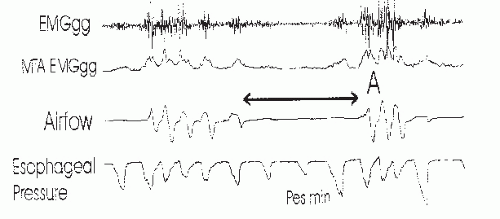
79). Of note, obstructive apnea may occur before the nadir in inspiratory effort is reached. If one monitors esophageal pressure in patients with OSAHS, the nadir in deflections in some patients can occur two or three breaths into the apnea (
Fig. 18-8) (
5,
13).
During REM sleep, ventilation is irregular, even in normal persons with the greatest irregularity during periods of phasic eye movements (
80). As periods of REM sleep are longest and the REM density (number of eye movements per time) is the highest during the early morning hours, it is not surprising that this is the time of the greatest changes in ventilation during sleep. Although the diaphragm is not affected by the generalized muscle hypotonia of REM sleep, periodic decrements in diaphragmatic activity are associated with periods of reduced tidal volume. Upper-airway muscles are also affected during REM sleep. In normal persons, during REM sleep, genioglossus tonic activity is reduced but phasic activity can still be detected if intramuscular electromyography (EMG) electrodes are used (
Fig. 18-9). During bursts of eye movements, both diaphragmatic and genioglossus phasic activity is often decreased (
81). REM sleep without phasic eye movements is called “tonic REM” and with eye movements “phasic REM.” The latter is associated with more breathing irregularity. Many patients with OSAHS have a much higher AHI during REM sleep or have events only during REM (REM-related OSAHS). However, two studies have found no greater collapsibility of the upper airway during REM than NREM sleep (
67,
68). This variance with clinical experience could be due to either the nonhomogeneous nature of REM or the fact that drops in diaphragm activity are also important for inducing apnea and hypopnea.
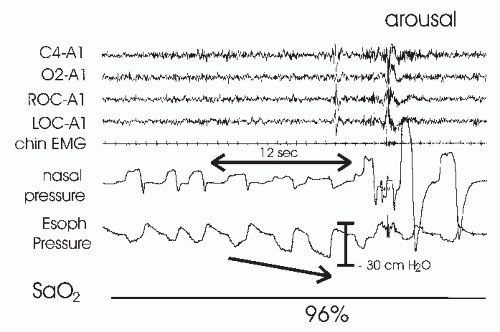
Mechanisms of Apnea Termination and Arousal
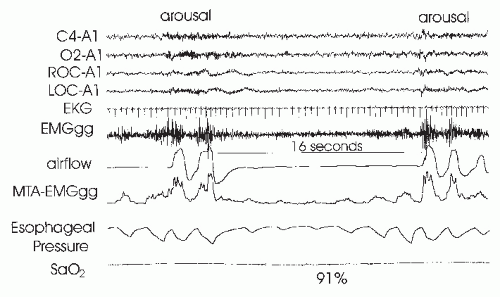
Obstructive apnea or hypopnea termination is believed to depend on arousal mechanisms. During obstructive apnea in NREM sleep, upper-airway muscle activity increases, as does inspiratory effort. Airway opening
does not occur until there is a preferential increase in upper-airway muscle activity (
4). This is often associated with signs of cortical arousal, although the electroencephalography (EEG) changes may not meet AASM criteria (
16), for example, the sudden onset of delta activity A recent study suggested that arousal can be detected at the termination of the majority of respiratory events if frontal electroencephalographs, as well as central EEG, is monitored (
82). If cortical arousal does not occur, there is still believed to be a “state” change in the brainstem—socalled subcortical or autonomic arousals. The term autonomic arousal is used because an abrupt change in heart rate or blood pressure can be detected at apnea termination, even if cortical arousal is absent. While hypercapnia and hypoxia drive the increase in respiratory effort, the level of effort rather than individual values of hypoxia or hypercapnia seems to trigger arousal (
13,
83). Thus, the level of effort is an index of the combined arousal stimulus. Studies have suggested that information from upperairway mechanoreceptors may contribute to the arousal stimulus (
84,
85). In NREM sleep, arousal appears to occur when inspiratory effort reaches an “arousal threshold.” Normal subjects tend to arouse during mask occlusion when suction pressure reaches 20- to 40-cm H
2O. In contrast, many patients with OSAHS arouse only after pressure reaches -60 to -80 cm H
2O (
13). The increased arousal threshold in OSAHS patients is probably due, in part, to chronic sleep deprivation or hypoxemia. Withdrawal of CPAP for even three nights has been shown to increase the arousal threshold in OSAHS. However, chronic CPAP treatment does not restore the arousal threshold to normal (
86). Patients with OSAHS could have an intrinsically increased respiratory arousal threshold. Alternatively there could be damage to mechanoreceptors from years of snoring or to chemoreceptors from repetitive nightly stimulation. A study of respiratoryrelated evoked potentials (RREP) in patients with mild OSAHS suggested that there is a sleep-specific blunted cortical response to inspiratory occlusion (
87). At least in these milder patients, there was no evidence of impaired mechanoreceptor function as the respiratory-related evoked potential was normal during wakefulness. One study found that the prolongation in event duration that occurs overnight in patients with OSAHS is secondary to a blunting of the cortical response as the level of inspiratory effort at apnea termination increased during the night (
88). Another study found that the within-night variation in the arousal threshold followed the cycles of NREM sleep (
89) with a higher arousal threshold associated with higher EEG delta power (deeper sleep).


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access