(1)
Assumptions of Lin et al.’s Algorithm
Lin et al. made several assumptions in their algorithm development. First, the contralesional hemisphere is unaffected by infarction and brain swelling. This implies that the measured volume of the contralesional hemisphere is the true volume and is typically a valid assumption. The second assumption is that swelling occurs only in the infarcted tissue, thus the area/volume of the nonischemic ipsilesional tissue is unaffected (i.e., the healthy ipsilesional tissue is its true size). This assumption makes Lin et al.’s algorithm vulnerable to artifacts caused by volume changes in the ipsilesional hemisphere, namely peri-infarct edema [4] and stunted brain growth.
Development of a New Algorithm for Calculating Infarct Volume
To better correct for the effects of brain hemisphere volume changes on infarct volume estimation, a new algorithm has been developed that relies on the ratio of the infarction to the whole ipsilesional hemisphere to estimate infarct area.
First, the infarct area is calculated by taking the difference in the area of the ipsilesional hemisphere and the non-infarcted ipsilesional hemisphere tissue, N i, for slice i, or 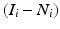 . Taking this difference removes bias of the analyzer towards the infarct area. This difference (infarct area) is then normalized to the area of the ipsilesional hemisphere for slice i, yielding the fractional amount of the ipsilesional hemisphere that is infarcted per slice, or
. Taking this difference removes bias of the analyzer towards the infarct area. This difference (infarct area) is then normalized to the area of the ipsilesional hemisphere for slice i, yielding the fractional amount of the ipsilesional hemisphere that is infarcted per slice, or
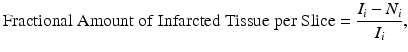
where I i is the ipsilesional hemisphere for slice i, N i is the noninfarcted ipsilesional tissue for slice i, and 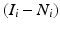 is the infarct area for slice i. To obtain the corrected area of the infarct, the fractional amount of infarcted tissue (Eq. 2) is multiplied by the area of the contralesional hemisphere for slice i (C i), or
is the infarct area for slice i. To obtain the corrected area of the infarct, the fractional amount of infarcted tissue (Eq. 2) is multiplied by the area of the contralesional hemisphere for slice i (C i), or
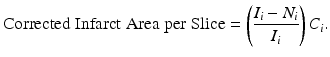
The corrected infarct area is then summed over all slices and multiplied by the thickness of each slice, d. To determine the percentage of the whole brain that is infarcted, the infarct volume is divided by two times the volume of the contralesional hemisphere. Thus, the novel algorithm for computing infarct volume is
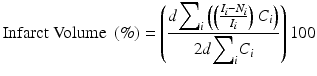
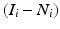 . Taking this difference removes bias of the analyzer towards the infarct area. This difference (infarct area) is then normalized to the area of the ipsilesional hemisphere for slice i, yielding the fractional amount of the ipsilesional hemisphere that is infarcted per slice, or
. Taking this difference removes bias of the analyzer towards the infarct area. This difference (infarct area) is then normalized to the area of the ipsilesional hemisphere for slice i, yielding the fractional amount of the ipsilesional hemisphere that is infarcted per slice, or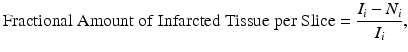
(2)
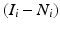 is the infarct area for slice i. To obtain the corrected area of the infarct, the fractional amount of infarcted tissue (Eq. 2) is multiplied by the area of the contralesional hemisphere for slice i (C i), or
is the infarct area for slice i. To obtain the corrected area of the infarct, the fractional amount of infarcted tissue (Eq. 2) is multiplied by the area of the contralesional hemisphere for slice i (C i), or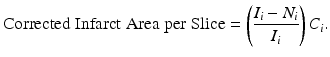
(3)
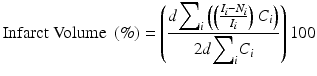
(4)
Assumptions of Our Algorithm
First, identical to the assumption made by Lin et al., the contralesional hemisphere is assumed to be unaffected by brain swelling, or the area of the contralesional hemisphere is the true area. Second, swelling occurs not only in the infarction but also in the peri-infarct region. This assumption relaxes the constraint of swelling only the infarct, made by Lin et al., which caused a majority of Lin et al.’s algorithm’s artifacts to exist.
Methods
All experiments were approved by Loma Linda University Institutional Care and Use Committee. Fifty-one adult male Sprague-Dawley rats (270–290 g) were distributed into middle cerebral artery occlusion (MCAO) (n = 24) or MCAO sham surgery groups (n = 27). Forty-four postnatal day 10 (P10) Sprague-Dawley rat pups were distributed into hypoxia-ischemia (HI) (n = 24) or HI sham surgery groups (n = 20).
Middle Cerebral Artery Occlusion Model
Two hours of MCAO was performed as previously described [20]. Briefly, anesthetized animals (ketamine (80 mg/kg) and xylazine (20 mg/kg), intraperitoneally) had the right common, internal, and external carotid arteries isolated. The external carotid artery was ligated, leaving a 3–4 mm stump. A 4.0 monofilament nylon suture with a rounded tip was inserted into the external carotid artery stump, advanced up through the internal carotid artery, and stopped when resistance was felt. After 2 h of occlusion, the suture was removed, beginning reperfusion. Sham surgery involved all procedures except for suture insertion and occlusion. One day after reperfusion, deeply anesthetized animals were sacrificed. Brains were removed, sectioned into 2 mm thick slices, placed into 2 % 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) for 15 min at room temperature, and then photographed.
Hypoxia-Ischemia Model
HI was performed as previously described [5, 7, 30]. Briefly, anesthetized P10 pups (isoflurane, 3 % induction, 2.5 % sustained, administered in a 100 % oxygen (0.3 mL/min)/medical gas (0.7 L/min) mixture), had the right common carotid artery exposed, isolated, and ligated using 5–0 silk surgical suture. Surgery time was controlled to be less than 5 min [7]. One hour after surgery, animals were placed into a hypoxia chamber for 2.5 h (8 % O2 and 92 % N2, maintained at 37 °C using a temperature-controlled water bath). The gas was delivered at a flow rate of 93.82 mL/min for the first 1.25 h and 77.30 mL/min for the remaining 1.25 h. After 2.5 h in the hypoxia chamber, animals were returned to their dams. Sham animals underwent identical surgical procedures, except for artery ligation and hypoxia (animals were exposed to normoxic conditions for 2.5 h). Two days after HI, deeply anesthetized animals were sacrificed. Brains were removed, sectioned into 2 mm thick slices, and placed into 2 % TTC for 15 min at room temperature. Stained slices were photographed.
Ipsilesional Hemisphere Volume
Infarct Volume
Statistical Analysis
Data is given as the mean ± standard deviation. Ipsilesional hemisphere volume data was analyzed using unpaired t-tests. Infarct volume data was analyzed using paired t-tests and F-tests.
Results
Ipsilesional Hemisphere Volumes
Animals subjected to MCAO presented with greater ipsilesional hemisphere volumes (ipsilesional hemisphere swelling) compared with that of sham animals (p < 0.001) (Fig. 1a). Animals subjected to HI had significantly smaller ipsilesional hemisphere volumes than those of sham animals (p < 0.001) (Fig. 1b).
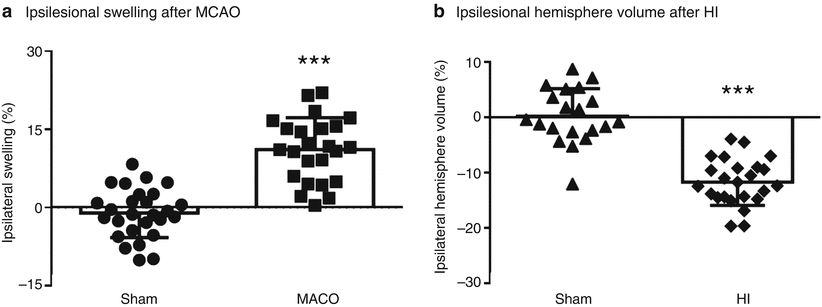

Fig. 1
Ipsilesional hemisphere volumes after ischemic stroke. (a) Ipsilesional swelling for animals subjected to MCAO (n = 24) or MCAO sham surgery (n = 27). (b) Ipsilesional hemisphere volume (%) for neonates subjected to HI (n = 24) or HI sham surgery (n = 20). *** p < 0.001 vs sham
Infarct Volume Comparison of Lin et al.’s Algorithm and Our Algorithm
Infarct volume computed using Lin et al.’s algorithm was statistically greater for MCAO animals than that of sham animals (p < 0.001). Similarly, the infarct volume calculated using our algorithm was significantly greater for MCAO animals than that of sham animals (p < 0.001). However, the infarct volumes of MCAO sham animals were significantly different between Lin et al.’s and our algorithms (F-test p < 0.001) (Fig. 2a). Additionally, the infarct volumes of MCAO animals were significantly different between the two algorithms (paired t-test p < 0.001) (Fig. 2b).
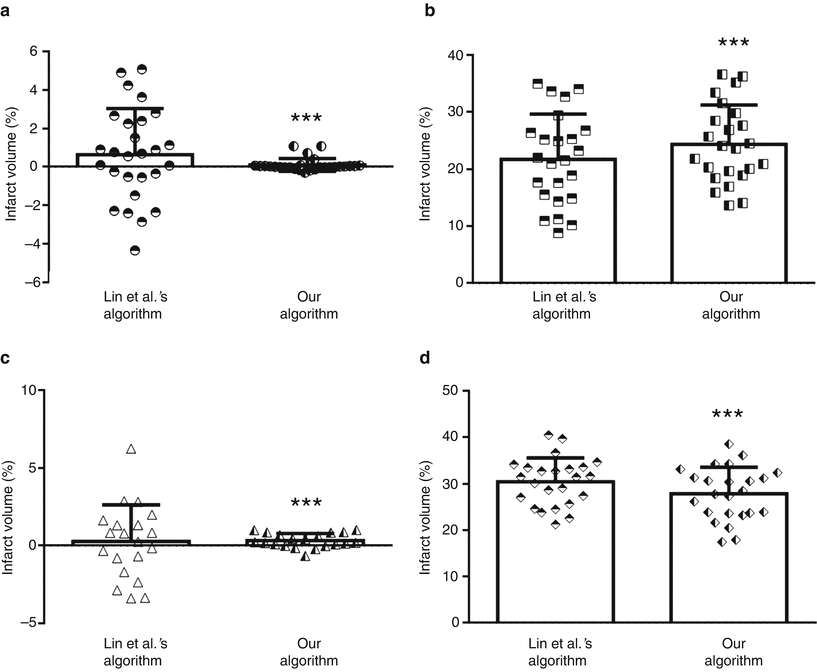

Fig. 2
Comparison of infarct volumes computed using Lin et al.’s algorithm and our algorithm. Infarct volumes of MCAO sham animals (a) (n = 27), animals subjected to MCAO (b) (n = 24), HI sham neonates (c) (n = 20), and neonates subjected to HI (d) (n = 24). ***p < 0.001 vs sham
For the algorithm by Lin et al., the infarct volume was significantly greater for HI animals than that of sham animals (p < 0.001). Similarly, for our algorithm, the infarct volume was statistically greater for HI animals than that of sham animals (p < 0.001). The infarct volumes of HI sham animals were significantly different between Lin et al.’s and our algorithm (F-test p < 0.001) (Fig. 2c). The infarct volumes of HI animals were significantly different between the two algorithms (paired t-test p < 0.001) (Fig. 2d).
Discussion
Herein, a novel algorithm to compute infarct volume after experimental stroke is described. The new algorithm changes one of the assumptions made for Lin et al.’s algorithm, thus reducing the effects of several ipsilesional hemisphere volume artifacts that impact the infarction volume computed by Lin et al.’s algorithm. The infarct volumes obtained via Lin et al.’s algorithm and our algorithm were compared using data from two ischemic stroke models, and in both models, Lin et al.’s algorithm was more affected by brain hemisphere volume artifacts (Fig. 3).
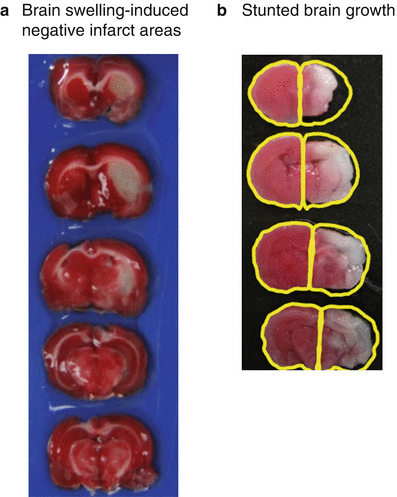

Fig. 3
Artifacts caused by changes in ipsilesional hemisphere volumes. (a) Representative MCAO brain for which brain swelling causes negative infarct areas. Table 1 contains the brain areas and the infarct areas for each slice. Ipsilesional swelling is 12 %. (b) Representative HI brain for which the ipsilesional hemisphere is smaller than that of the contralesional hemisphere. This difference leads to negative ipsilesional hemisphere brain volumes (ipsilesional hemisphere volume is −16 %)
Limitations of Lin et al.’s Algorithm
The major limitations of Lin et al.’s algorithm arise from the second assumption: only infarction swelling occurs. This makes their algorithm vulnerable to artifacts, the most influential of which is brain hemisphere volume changes. The two major causes of hemisphere volume changes in experimental stoke models are brain swelling and stunted brain growth; the former being primarily observed in adult experimental stroke models and the latter primarily observed in neonatal experimental stroke models.
Artifact 1: Negative Infarct Areas Caused by Brain Swelling
Adult rats subjected to MCAO are prone to hemispheric brain volume changes via brain swelling (Fig. 3a). While minimal swelling is corrected for in Lin et al.’s algorithm, severe brain swelling and peri-infarct swelling can result in altered infarct volume measurements, such that negative infarct areas can be obtained (Table 1). This occurs when the nonischemic ipsilesional area is greater than that of the contralesional area for slice i (i.e., 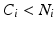 ).
).
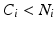 ).
).Table 1




Brain swelling-induced negative infarct areas
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








