Fig. 1
CpG protects the BBB in an in vitro model of ischemic injury. (a) Schematic diagram of in vitro BBB model consisting of brain microvascular endothelial cells (BMECs) co-cultured with glial cells. Pretreatment with CpG attenuates OGD-induced decrease of trans-endothelial-electrical resistance (TEER) (b) and increase of permeability for Na Fluorescein (c) in an in vitro BBB model of ischemic injury. Values are group means ± SEM; *p < 0.001 versus control (CTR) and °p < 0.01 versus OGD
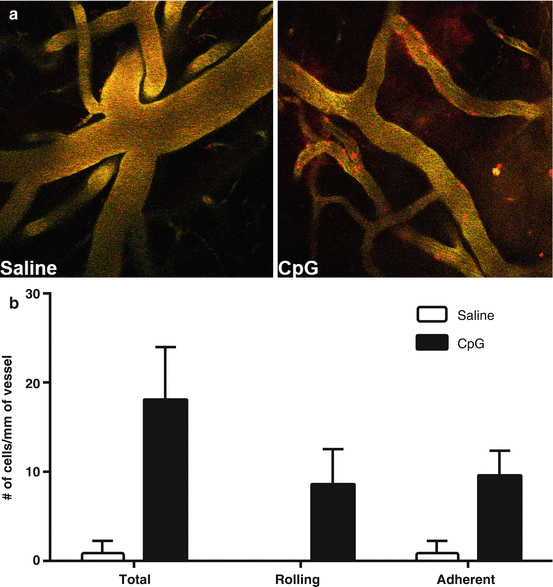
Fig. 2
CpG-induces leukocyte-endothelial interactions. (a) Representative images of cortical vessels in mice acquired by in vivo two-photon microscopy 24 h after treatment with saline or CpG (0.8 mg/kg). Leukocytes stained in red. (b) Quantification of leukocytes per mm of vessel from five separate video clips +/− SEM of an individual mouse for each treatment
It remains to be elucidated which leukocyte populations are required for CpG induction of neuroprotection. Bregs offer potential as they are known to be immunosuppressive, and CpG has been reported to drive the differentiation of B cells into Bregs [34]. In line with a protective role for Bregs prior to stroke, Bodhankar et al. [26] showed that adoptive transfer of Bregs 24 h before cerebral ischemia reduces infarct size. Mice receiving IL-10-secreting B cells showed an increase of regulatory cell populations in the periphery and reduced infiltration of T cells and proinflammatory cytokines levels in the ischemic hemisphere. These results show that the increased presence of Bregs before stroke can modulate the inflammatory response, potentially contributing to protection against subsequent ischemia. In further support, Monson et al. [7] found that repetitive hypoxic preconditioning (RHP) induces an immunosuppressive phenotype in resident B cells before stroke and results in decreased infiltration of leukocytes in the brain following stroke. It appeared that RHP reprograms B cells to downregulate genes involved in T cell differentiation and B-T cell interactions [7].
Conclusions
These studies clearly demonstrate the complex nature of the inflammatory response associated with stroke. The interplay between damaging and protective effects is a delicate balancing act that determines the extent of injury. Understanding this interplay and developing ways to modulate the immune cell effectors could greatly advance therapeutic treatment of ischemic brain injury, including stroke. Recent data suggest that endogenous mechanisms engaged by preconditioning include modulation of immune cells, highlighting the effectiveness of targeting these responses in mitigating cerebral ischemic injury.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
1.
2.
3.
Hurn PD et al (2007) T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab 27(11):1798–1805PubMedCentralCrossRefPubMed
4.
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808PubMedCentralCrossRefPubMed
5.
6.
Bodhankar S et al (2013) IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab Brain Dis 28(3):375–386PubMedCentralCrossRefPubMed
7.
Monson NL et al (2014) Repetitive hypoxic preconditioning induces an immunosuppressed B cell phenotype during endogenous protection from stroke. J Neuroinflammation 11:22PubMedCentralCrossRefPubMed
8.
Gelderblom M et al (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40(5):1849–1857CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








