Fig. 1
Brain water content (a), sodium (b), and potassium (c) concentrations in brains of female and male mice at 24 h after intracaudate injection of FeCl2 (1 mM in 10 μL saline). Values are mean ± SD, n = 8 or 14, *p < 0.05
Co-injection of ICI 182, 780 enhanced FeCl2-induced brain edema (80.3 ± 1.3 vs 79.3 ± 0.4 % in vehicle, p < 0.05, Fig. 2a), sodium accumulation (269 ± 66 vs 220 ± 28 μEq/g in vehicle, p < 0.05, Fig. 2b), and potassium loss (470 ± 48 vs 522 ± 29 μEq/g dry weight in vehicle, p < 0.01, Fig. 2c) in the ipsilateral hemisphere in females. Intracaudate injection of ICI 182, 780 (1 μg) did not induce brain edema formation (79.0 ± 0.2 vs. 79.1 ± 0.2 % in the contralateral hemisphere, p > 0.05).
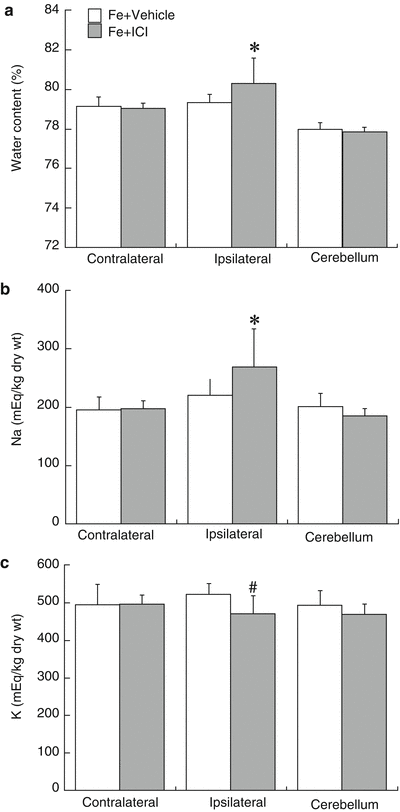

Fig. 2
Brain water content (a), sodium (b), and potassium (c) concentrations in female mice 24 h after intracaudate injection with FeCl2 (1 mM) mixed with ICI 182, 780 (1 μg) or vehicle. Values are mean ± SD, n = 9 to 11, *p < 0.05 and #p < 0.01
Systemic treatment with tamoxifen (5 mg/kg, IP) in males significantly reduced FeCl2-induced brain edema (78.7 ± 0.4 vs 81.1 ± 1.8 % in vehicle, p < 0.05, Fig. 3a). This reduced edema was associated with a reduction in sodium accumulation (188 ± 7 vs 331 ± 130 μEq/g dry weight, p < 0.05, Fig. 3b) and a tendency for reduced potassium loss (474 ± 18 vs 448 ± 34 μEq/g dry weight, p > 0.05, Fig. 3c) in the ipsilateral hemisphere.
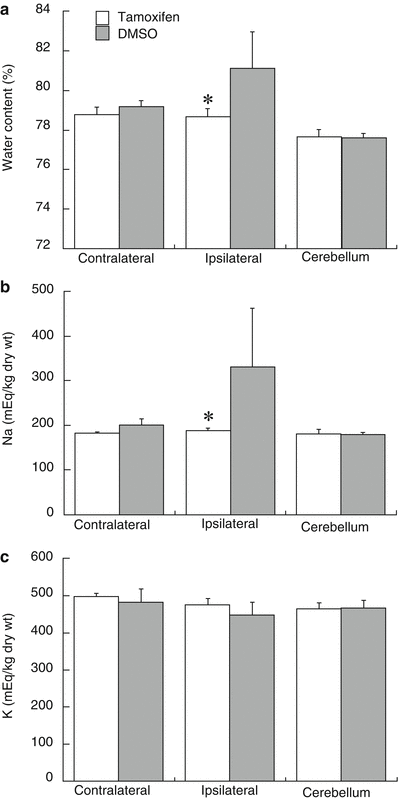

Fig. 3
Brain water content, sodium, and potassium concentrations of male mice 24 h after intracaudate injection with FeCl2 (1 mM, 10 μL) into right caudate. Mice were treated with tamoxifen (5 mg/kg) or DMSO intraperitoneally at 1 h after the injection. Values are mean ± SD, n = 5, *p < 0.05
Discussion
Our previous studies showed that estrogen pretreatment reduces iron-induced brain edema and neuronal death in rats [10]. In this study, we found that iron induced less brain edema formation in female mice than in male mice. The nonspecific ER antagonist, ICI 182, 780, enhanced iron-induced brain edema formation in females. We have previously shown that ICI 182, 780 exacerbates ICH-induced brain edema in female but not in male rats [20]. In addition, one systemic treatment once with a high dose of tamoxifen (5 mg/kg) at 1 h post could reduce iron-induced brain edema in males. These results all suggest a role of ER in reducing iron-induced brain injury (a component of ICH-induced injury).
Since Kimelberg et al. [14] reported a neuroprotective effect of tamoxifen in males after stroke, tamoxifen has been shown to have neuroprotective effects in females and in other diseases such as Parkinson’s disease [5, 14, 19]. We have shown that tamoxifen (5 mg/kg) is neuroprotective in male rats after ICH [32]. Estrogen-receptor modulators may have effects via genomic and non-genomic signaling and antioxidant mechanisms [4]. In support of a direct antioxidant action, tamoxifen has been shown to have direct superoxide scavenging abilities in vitro [17]. However, whether the direct antioxidant action of tamoxifen involves activation of the estrogen receptors remains unclear. Iron induces lipid peroxidation and free radical formation, which contributes to delayed edema along with oxidative stress after ICH [12, 25, 28, 31]. The effects of tamoxifen on iron-induced brain edema may have similar mechanisms to those inducing neuroprotection in cerebral ischemia, but this needs further investigation. It should be noted that there was a study showing that ICI 182, 780 administered did not block the effect of high-dose tamoxifen on infarct size, which suggests that tamoxifen may act in an estrogen receptor-independent manner [33].
In conclusion, there are remarkable gender differences in iron-induced brain edema. This protection in female mice can be blocked by a nonspecific estrogen receptor antagonist, ICI 182,780. In addition, the estrogen receptor modulator tamoxifen attenuated iron-induced brain edema in male mice. The results from this study suggest a role of estrogen receptors in reducing iron-induced brain edema.
References
1.
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5:472–475PubMedCentralCrossRefPubMed
2.
Culmsee C, Vedder H, Ravati A, Junker V, Otto D, Ahlemeyer B, Krieg JC, Krieglstein J (1999) Neuroprotection by estrogens in a mouse model of focal cerebral ischemia and in cultured neurons: evidence for a receptor-independent antioxidative mechanism. J Cereb Blood Flow Metab 19:1263–1269CrossRefPubMed
3.
Davis CM, Fairbanks SL, Alkayed NJ (2013) Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res 4:381–389PubMedCentralCrossRefPubMed
4.
5.
Dluzen DE, McDermott JL, Anderson LI (2001) Tamoxifen diminishes methamphetamine-induced striatal dopamine depletion in intact female and male mice. J Neuroendocrinol 13:618–624CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








