Fig. 1
(a) Representative T2 MRI scans of the brains of female and male LCR rats 24 h after an intracaudate injection of FeCl2. (b) Lesion volumes calculated from such MRI scans. (c) Brain swelling calculated from such scans. Male LCR rats had significantly greater lesion volumes and brain swelling than females after FeCl2 injection. Values are mean ± SD, n = 6–7, *p < 0.05
Albumin is normally excluded from the brain by the blood-brain barrier (BBB), and entry of albumin is a marker of BBB disruption. Intracaudate FeCl2 injection caused marked BBB disruption in the ipsilateral hemisphere 24 h after injection (Fig. 2) with much higher albumin protein levels in the ipsilateral basal ganglia than in the contralateral (p < 0.01, Fig. 2). The albumin-positive area was larger in the ipsilateral hemisphere in males than females (Fig. 2) and albumin protein levels (Western blot) were significantly higher in males (7,717 ± 1,502 vs 5,287 ± 1,342 pixels in females, p < 0.05; Fig. 2).
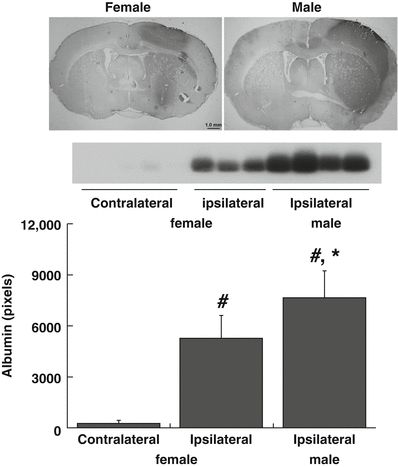

Fig. 2
Albumin immunohistochemistry and Western blot demonstrating protein extravasation into brain 24 h after intracaudate injection of FeCl2 in female and male LCR rats. Albumin extravasation is a marker of BBB disruption. Bar graph quantifies the Western blot data. FeCl2 injection caused unilateral BBB disruption that was significantly greater in male rats. Values are mean ± SD, n = 3–4, *p < 0.05 male vs female; #p < 0.01 ipsi- vs contralateral
HO-1 is a marker for brain stress that is expressed at very low levels in normal brain. The number of cells immunoreactive for HO-1 at 24 h after FeCl2 injection was greater in male than female LCR rats (Fig. 3). Similarly, as assessed by Western blot, HO-1 protein levels in the ipsilateral basal ganglia were higher in males than females (HO-1/β-actin: 1.31 ± 0.44 vs 1.03 ± 0.05, p < 0.05; Fig. 3).
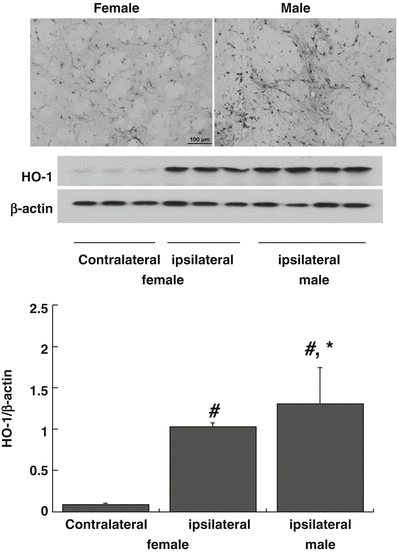

Fig. 3
Heme oxygenase 1 (HO-1) immunohistochemistry (ipsilateral caudate) and Western blot (basal ganglia) 24 h after intracaudate injection of FeCl2 in LCR and HCR rats. The bar graph quantifies the Western blot normalizing the data to β-actin. FeCl2 injection caused unilateral HO-1 upregulation and that upregulation was significantly greater in male rats. Values are mean ± SD, n = 3–4, *p < 0.05 male vs female; #p < 0.01 ipsi- vs contralateral
Discussion
In the present study, we found that male rats had comparatively more severe hemispheric swelling, BBB disruption, and larger T2 lesions after intracerebral iron injection into the caudate. In addition, iron induced much stronger expression of HO-1 in males than females.
Brain iron overload has an important role in ICH-induced brain injury. The current results suggest that differences in iron-mediated damage contribute to gender differences in ICH-induced brain injury. It is still unknown why iron-induced brain swelling is less in female LCRs. For iron-induced brain injury, most research has focused on oxidative injury. The reduction in the iron-induced upregulation of brain HO-1 expression (a cellular stress marker) in females may reflect reduced oxidative stress. This needs investigation.
Iron-induced brain edema could be vasogenic and cytotoxic. Our results showed that BBB leakage was more severe in males than females, suggesting less vasogenic brain edema in females. We have previously shown that females have less severe ICH-induced brain injury than males through an estrogen receptor-dependent mechanism [9]. More experiments are needed to determine whether estrogen and its receptors have a role in ameliorating iron-induced BBB disruption and vasogenic brain edema.
In conclusion, iron caused less brain damage, BBB leakage, and brain swelling in female LCRs compared with males. Gender differences in iron-induced injury may contribute to differences between females and males in ICH-induced brain injury.
Acknowledgments
This study was supported by grants NS-073959, NS079157, and NS-084049 from the National Institutes of Health (NIH) and 973 Program-2014CB541600. The LCR-HCR rat model system was funded by the Office of Research Infrastructure Programs/OD grant R24OD010950 and by grant R01DK099034 (to LGK and SLB) from the National Institutes of Health. SLB was also supported by National Institutes of Health grants R01DK077200 and R01GM104194. We acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Heckenkamp. Contact LGK (lgkoch@med.umich.edu) or SLB (brittons@umich.edu) for information on the LCR and HCR rats: these rat models are maintained as an international resource with support from the Department of Anesthesiology at the University of Michigan, Ann Arbor, Michigan.
References
1.
Davis CM, Fairbanks SL, Alkayed NJ (2013) Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res 4:381–389PubMedCentralCrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








