Fig. 1
a Left Diagram showing the putative transmembrane topology of nAChR subunits. The extracellular amino terminal portion is followed by three hydrophobic transmembrane domains (M1–M3), a large intracellular loop, and then a fourth hydrophobic transmembrane domain (M4). Middle Pentameric arrangement of nAChR subunits in an assembled receptor. Right Localization and schematic organization of the ACh binding sites in a heteromeric receptor. b Structure of heteropentameric and homopentameric neuronal subtypes. The pentameric arrangement of nAChR subunits in an α7 homopentameric subtype (right), heteromeric receptor subtypes (middle), and the (α4)3(β2)2 subtype (right). The localisation of the subunit interfaces of the orthosteric binding sites is indicated, with the primary component (P) carried by the α subunits, and the complementary component (C) carried by α or non-α subunits. In addition to the two orthosteric sites, the (α4)3(β2)2 subtype has a binding site at the α4/α4 interface (star)
In vertebrates the genes that have been cloned so far (CHRNA2–CHRNA10, and CHRNB2–CHRNB4) code for subunits that are divided into two subfamilies of nine α subunits (α2–α10) and three β (β2–β4) expressed in the nervous system, cochlea, and a number of non-neuronal tissues (Gotti and Clementi 2004). All the nine α subunits have adjacent cysteines (analogous to cysteines 192–193 of the α subunit of the muscle-type AChR), whereas the β subunits do not have cysteines. The different combinations of nAChR subunits have led to the formation of a heterogeneous family of pentameric subtypes with different structural, functional, and pharmacological properties. Two main classes of nAChR subtypes have been identified: the αBgtx-sensitive receptors, which are made up of the α7, α8, α9 and/or α10 subunits can form homomeric or heteromeric receptors, and the αBgtx-insensitive receptors, which are heteromeric receptors, formed by α and β subunits that bind agonists with high affinity but not αBgtx (Fig. 1c) (reviewed in Gotti et al. 2009).
Studies of heterologous systems have shown that α7 subunits can also form functional channels with the subunits present in non-αBgtx binding receptors such as the α5 (Girod et al. 1999), β2 (Khiroug et al. 2002), β3 (Palma et al. 1999) and β4 subunits (Criado et al. 2012). It is presumed that both homomeric and heteromeric nAChRs have a pentameric structure with the subunits organized around a central channel: the homo-oligomeric receptors have five identical (orthosteric) ACh binding sites per receptor molecule (Fig. 1b) (Palma et al. 1996) located at the interface between two adjacent subunits, whereas hetero-oligomeric receptors have two or three α subunits and three or two β subunits, and therefore two orthosteric binding sites per receptor molecule located at the interface between the α and β subunits (Taly et al. 2009) (see Fig. 1b). Each orthosteric ACh binding site has a principal (or ‘‘plus’’) and a complementary or ‘‘minus’’ component. In heteromeric nAChRs, the principal component is carried by the α2, α3, α4, and α6 subunits with the complementary site carried by the β2 or β4 subunits, whereas each subunit in the homomeric receptors contributes to both the principal and complementary components, which are present on opposite sides of the same subunit (reviewed in Corringer et al. 2000; Taly et al. 2009) (see Fig. 1b).
Heteropentamer neuronal nAChRs have the fifth subunit that does not contribute to the orthosteric site (and this subunit is called accessory subunit). In heterologous systems, α5 and β3 subunits only form functional channels when they are co-expressed with a primary and complementary subunit (Groot-Kormelink et al. 1998; Ramirez-Latorre et al. 1996) thus indicating that they can only function as accessory subunits, whereas the α3 or α4 and β2 or β4 subunits can form orthosteric ligand binding sites or assemble in the accessory position to produce receptors with different stoichiometries (see Fig. 1b).
However, recent studies have revealed further complexity in the definition of binding sites and the possible subunit involved. The use of concatenated receptors whose DNA was linked covalently led to the expression of receptors whose subunits were in a specified order, and whose pharmacological properties were similar to those of the non-linked receptors (Nelson et al. 2003; Zhou et al. 2003). Studies of the concatameric (α4)3 (β2)2 subtype have shown that in addition to the two orthosteric binding sites at the α4/β2 interface they also have an additional binding site at the α4/α4 interface (Moroni et al. 2008) as shown in Fig. 1b left.
Jin et al. (2014) have very recently, using the concatameric approach, expressed either the dimeric constructs of α4 and β2 subunits expressed with a free α5 subunit and concatameric pentameric, receptors incorporating a single copy of α5, in different positions, and found that the α5 subunit can occupy the position of a non-binding subunit, or replace a β2 subunit participating in an orthosteric binding site. However, functional receptors apparently cannot be formed with α5 subunits in both canonical binding sites.
2.1 Orthosteric ACh Binding Sites
Much of our knowledge of agonist binding sites comes from studies of muscle AChRs in which the use of affinity labelled reagents and subunit chimeras and/or site-directed mutagenesis have shown that it is the large extracellular amino terminal domain that contributes to the ACh binding domain pocket (Bartos et al. 2009; Taly et al. 2009). Moreover, recently a significant contribution to the identification of the ligand binding site in nAChRs has also been made by analyzing the crystal structure of the ACh binding protein from the freshwater snail (Celie et al. 2005; Rucktooa et al. 2009). This homopentameric soluble protein (AChBP), which is 210 residues long, binds ACh and is secreted by snail glial cells into cholinergic synapses and has an affinity spectrum resembling that of homomeric α7 or α9 receptors.
Many amino acid residues contribute to the orthosteric ACh binding site. They are grouped into short sequences that form loops A, B, and C (the principal component) and D, E, and F (the complementary component) (Fig. 2a). Inside the compact structure of an AChBP, at the center of the interface of the ligand binding domain (LBD) of two adjacent subunits, loops A, B, D, and F form a hydrophobic pocket to which agonists bind that is closed by loop C (Fig. 3d). Studies of the co-crystals of AChBPs using nicotinic ligands have shown that the conserved residues in the binding site are tyrosine (Y) 93 (loop B), tryptophan 149 (W) and Y151 (loop A), Y190 and Y198 (loop C), W55 and E57 (loop D), and the disulfide bridge between cysteines (C) 192 and 193 (the numbering refers to the amino acids present in Torpedo AChRs) (Changeux and Taly 2008). The aromatic residues and disulfide bridge of the ACh binding site are electronegative and neutralize the positive charge carried by the majority of nicotinic ligands. The binding of ACh therefore gives rise to a non-covalent interaction between the cation and the electron-rich π system of the W residue in loop B. In general, the tertiary or quaternary ammonium charges of nicotinic ligands bind to the center of the pocket formed by the hydrophobic aromatic residues of loops A, B, C, and D. This interaction is maintained in the family of Cys-loop receptors, although the loops involved depend on the receptor type (Miller and Smart 2010).
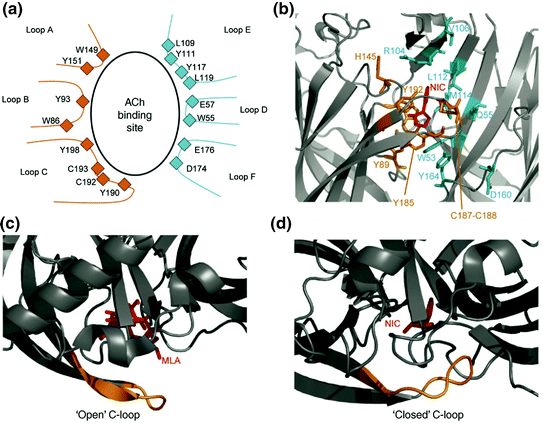
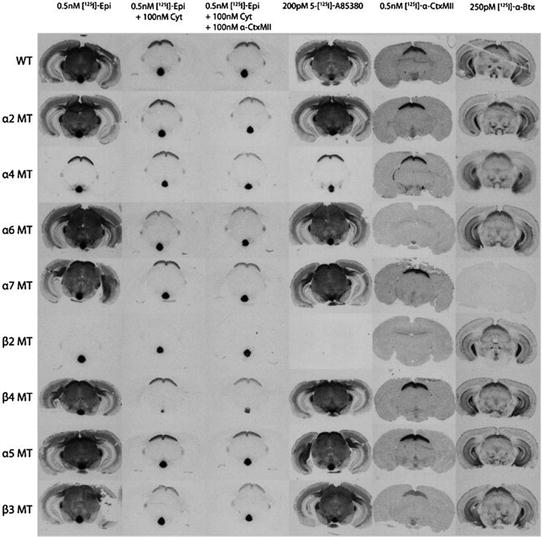

Fig. 2
Structure of the ACh binding site. a Diagram of an ACh binding site showing the amino acids in the loops that participate in its formation. Loops A, B, C are provided by the α subunit, and loops D, E, F by the adjacent subunit. b–d The X-ray structure of the binding site of the AChBP co-crystallographed in the presence of nicotine (b and d) and the antagonist methyllycaconitine (MLA) (c). Agonists bind in a pocket containing the binding site that promotes extensive contact between the ligand and protein. As in the case of other antagonists, the binding of methyllycaconitine leads to an open conformation of loop C (yellow) that interferes with receptor activation and channel opening (c). Figure is reproduced with permission from (Changeux and Taly 2008)

Fig. 3
Autoradiographic images of coronal mouse brain sections at approximately −3.5 μm Bregma. Autoradiograms of 125I-epibatidine (total binding, binding in the presence of 100 nM cytisine and binding in the presence of 100 nM cytisine and 100 nM αconotoxin MII), 125I-A85380, 125I-α-conotoxin MII and 125Iα-Bungarotoxin to WT mice and each of the nAChR KO mice for sections at a level of approximately 3.5 mm Bregma. Figure is reproduced with permission from (Baddick and Marks 2011)
X-ray studies of AChBPs have shown that, in the absence of the agonist or presence of the antagonist, loop C does not cover the hydrophobic pocket, whereas in the presence of the agonist, the binding site has a closed conformation with loop C covering it (Figs. 3c, d). The role of the movement of loop C in activating the receptor was determined by the studies of a chimera obtained from the fusion of most AChBPs to the transmembrane regions of the serotonin (5-HT3) receptor. This chimera functions as an ACh receptor in which ACh binding determines the closure of loop C and the activation of the receptor (Mukhtasimova et al. 2009).
An electrophysiology study has also identified feedback communication between the movement of loop C and the opening of the channel. This study also demonstrated that the binding of one molecule of ACh opens the channel for a short time, whereas the binding of two molecules prolongs the opening (Mukhtasimova et al. 2009). It seems, therefore, that the closed conformation of loop C gives rise to a state of channel pre-activation and that the duration of channel opening depends on the number of closed conformations of loop C in the receptor. Single-channel analyses of ACh receptors has shown that full agonist binding easily allows the transition from pre-activation to complete activation of the receptor and the consequent closing of loop C.
2.2 Accessory Subunits
In heteromeric αBgtx-insensitive receptors, the accessory subunits are those that do not directly participate in forming the binding site. The role of accessory subunit has been investigated in the α4β2*1 subtypes in which the presence of different accessory subunits (α5, β3, α4, β2) changes their pharmacological and biophysical properties, their sensitivity to allosteric modulators, and their sensitivity to up-regulation (Kuryatov et al. 2008; Moroni et al. 2006, 2008; Tapia et al. 2007).
The (α4β2)2α5 subtype has the highest Ca2+ permeability, whereas the (α4β2)2β2 subtype has the greatest affinity for ACh and nicotine activation, and is also the most sensitive to nicotine desensitization (Kuryatov et al. 2008). Moreover, the presence of the α5 subunit in the α4β2* subtype confers sensitivity to the allosteric modulator galantamine (Kuryatov et al. 2008). The inclusion of the α5 subunit also affects the pharmacological and functional properties of other subtypes: for example in the α3β2* and α3β4* subtypes, it increases desensitization and Ca2+ permeability, and alters agonist-stimulated responses (Tapia et al. 2007).
The β3 subunit co-assembles with several nAChR subunit combinations, but in all cases other than α3β3β4, it appears to have a dominant negative effect that leads to the absence of the functional expression of the assembled β3* receptor complex (Broadbent et al. 2006; Palma et al. 1999). However, our ex vivo studies (Gotti et al. 2006) indicate the great propensity of β3 to assemble with α6 subunit, and α6* receptor expression in β3 knockout mice is decreased in the cell bodies and nerve terminals of dopaminergic neurons. This decrease suggests that the β3 subunit is important for the formation of the majority of α6β2* or α4α6β2* receptors, and that its loss causes defects in nAChR assembly, degradation, and/or trafficking. The exclusive role of the β3 subunit as an accessory subunit has been confirmed using fluorescently labelled α6 and β3 subunits and the FRET technique, which has shown that only a single β3 subunit is incorporated in pentameric α6β2* receptors (Drenan et al. 2008). So the accessory subunit in the fifth position may influence many characteristics of nAChRs including their agonist sensitivity, channel kinetics, Ca2+ permeability, assembly, interactions with chaperone proteins, trafficking, and cell localization (reviewed in Colombo et al. 2013).
2.3 Subunit Stoichiometry
As neuronal nAChRs are pentameric, they can have considerable molecular differences in subunit composition or different subunit stoichiometries even if they have the same subunit composition. Heterologously expressed α4β2 and α3β4 subtypes can exist in two stoichiometries, made of either two or three copies of the α subunit in the channel pentamer. The two stoichiometries of the α4β2 subtype can be distinguished because the subtype with two α4 subunits, (α4)2(β2)3 subtype, is activated at much lower ACh concentrations and is more sensitive to other agonists than the subtype with three α4 subunits, (α4)3(β2)2 subtype (Moroni et al. 2006). Nicotinic ligands may also have different efficacy toward the two stoichiometries: for example, the selective α4β2 ligand sazetidine binds with high affinity to both stoichiometries but it is a full agonist of (α4)2(β2)3 nAChRs, while had an efficacy of only 6 % on(α4)3(β2)2 nAChRs (Zwart et al. 2008). The agonist sensitivity of the two α3β4 stoichiometries is similar, but only the subtype with two α3 subunits is susceptible to enhancement by low zinc concentrations, and the two stoichiometries have markedly different single-channel conductance and kinetics (Krashia et al. 2010).
The two stoichiometries (α4)2(β2)3 and (α4)3(β2)2 exist functionally in rodent cortical and thalamic brain preparations (Gotti et al. 2008), whereas the different α4β2 and α3β4 stoichiometries expressed in heterologous systems depend on the expression system (Krashia et al. 2010; Moroni et al. 2006).
Recent studies have shown that the stoichiometries of the α4β2 and α3β4 subtypes are both modulated by exposure to nicotine , mainly by acting intracellularly during α4β2 receptor assembly, nicotine favors the formation of the (α4)2 (β2)3 stoichiometry, which is up-regulated both intracellularly and at the plasma membrane of heterologous cells and neurons (Colombo et al. 2013).
Nicotine also changes the stoichiometry of the α3β4 subtype. Recent data from our laboratory have shown that, during α3β4 assembly, nicotine binding favors an (α3)2(β4)3 stoichiometry that is more stable and preferably released from the endoplasmic reticulum for transport to the plasma membrane (Mazzo et al. 2013).
3 Native Subtypes
3.1 Techniques for Studying Native nAChRs
The approaches currently used to localize and identify nAChR subtypes include techniques for localizing subunit mRNA (in situ hybridization (ISH) and single-cell PCR) or protein (immunoprecipitation and immunocytochemistry), receptor autoradiography at regional or cellular level, techniques for assessing subtype composition and pharmacology (binding in tissue homogenates, immunoprecipitation, immunopurification, and Western blotting), and functional assays (neurotransmitter release from slices or synaptosomes and electrophysiological techniques).
Information concerning the subunit associations of most of the nAChR subtypes identified in neurons comes from immunoprecipitation and immunopurification studies using subunit-specific antibodies (Ab), whose specificity has been tested in the tissues of wild-type (WT) and knockout (KO) mice, and brain areas. This was possible because the native subtypes extracted from the membrane were radiolabelled with nicotinic ligands, and the selection of the antigens was made by receptor binding. The immunoprecipitation of native radiolabelled receptors also ensured that only pentameric receptors were immunoprecipitated because analysis of the sucrose gradients of the different native subtypes solubilized using a non-denaturing detergent (such as Triton x-100) shows that they preserve their pentameric assembly, unlike transfected subtypes in which high affinity binding sites can also be detected with a sedimentation coefficient that is not exclusively compatible with pentameric subunit assembly (Kuryatov et al. 2000).
The immunochemical localization of neurotransmitter receptors and microscopic subunit protein co-localization are important criteria when localizing native subtypes and defining their subunit composition . However, these techniques cannot be used in the case of nAChR subunits because most of the available anti-subunit antibodies (Abs) are nonspecific, which means that immunocytochemistry labelling leads to similar staining patterns in tissues obtained from the WT and KO mice (Moser et al. 2007).
The major drawback of immunoprecipitation and immunopurification is that the spatial resolution obtained is only at regional level.
In summary, although very few nicotinic ligands are subtype specific, the combined use of ligand binding assays and autoradiographic studies of brain tissues from WT and subunit KO mice has provided critical data for identifying and defining the subunit composition, localization, and pharmacology of native subtypes.
3.2 Autoradiographic Studies
The earliest autoradiographic ligand binding studies demonstrated that the binding of 125I-αBgtx was distinct from that obtained using the radioactive agonist ligands 3H-nicotine, 3H-ACh, and 3H-cytisine (Clarke et al. 1985; Marks and Collins 1982) and a number of studies showed that 3H-cytisine and 3H-ACh bind with nM affinity to the same sites bound by 3H-nicotine. Badio and Daly (1994) subsequently identified a new ligand epibatine that binds with very high affinity (pM) heteromeric receptors to the same sites as those bound by 3H-nicotine. Since then, further saturation binding studies to rat and chick membranes have shown that epibatidine also binds to low affinity sites and that some of this binding was competed by the presence of αBgtx (Marks et al. 2006).
Over the last 10 years many more ligands specific for the different subtypes have been discovered, including A85380 (which is specific for β2-containing receptors) and αconotoxin MII, which is specific for the α3β2 and α6β2 subtypes. The use of these old (125I-αBgt and 125I-epibatidine) and new ligands (125I-αconotoxin MII, and 125I-A85380) and brain slices obtained from WT and α2, α4, α6, α7, β2, β4, α5, and β3 KO mice has provided a clear picture of the localization of the subtypes (Baddick and Marks 2011) (see Fig. 3).
Deletion of the α7 subunit completely and selectively eliminates 125I-αBgtx binding, but deletion of any of the other subunits had no effect. Deletion of the β2 subunit completely eliminates 125I-A85380 binding throughout mouse brain, whereas deletion of the α4 subunit eliminates most 125I-A85380 binding, but residual 125I-A85380 binding sites were found in the dopaminergic pathways, visual tracts, medial habenula, and interpeduncular nucleus probably because of the presence of the α3β2* subtype. The binding of 125Iα-conotoxin MII is eliminated in most brain regions by deleting α6 or β2 subunit, and reduced by deleting the α4 or β3 subunit (Baddick and Marks 2011).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





