Chapter 6 Physiology of pain
INTRODUCTION
Despite the difficulty in arriving at an acceptable definition of pain, most people would agree that it can be of a variable quality, ranging from mild irritation, through itching, burning and pricking sensations to more intense stabbing and throbbing sensations and finally to agonizing, intractable pain, which for some subjects can be beyond endurance. In most cases these sensations are associated with the activation of nociceptors and the sensation of pain, but the differences in the subjective responses reflect both the strength and severity of the nociceptor activation and subjects’ individual psychological and emotional responses to this information. As will be discussed later, these differences can be important in the modulation of pain in certain circumstances.
PERIPHERAL ASPECTS
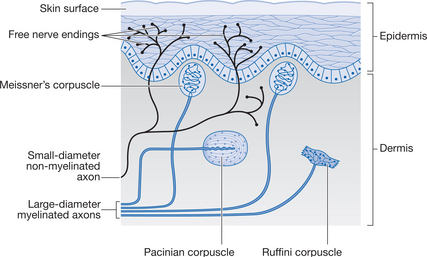
Nociceptors are generally free nerve endings embedded throughout the tissues; there are variations in the density of these receptors in different tissues. The free nerve endings are no more than simple nerve endings without any of the associated accessory structures that can be found with other sensory nerve endings (Fig. 6.1). The free nerve endings have a relatively high threshold to activation and are sensitive to potentially tissue-damaging stimuli such as mechanical, thermal, electrical and chemical stimuli.
These free nerve endings give rise to small-diameter afferent nerve fibres, which convey action potentials to the spinal cord and higher centres in the CNS. These afferent fibres are classed as either myelinated Aδ fibres, with conduction velocities of between 5 and 30m/s, or non-myelinated C fibres, which conduct action potentials at velocities between 0.5 and 2m/s. (Note: Aδ fibres are sometimes referred to as group III fibres and C fibres as group IV fibres.) These two types of afferent fibre are responsible for what is termed ‘fast’ and ‘slow’ pain, the properties of which are outlined in Table 6.1.
Table 6.1 Properties of ‘fast’ and ‘slow’ pain fibres
| Properties | Fast pain | Slow pain |
|---|---|---|
| Receptors | Free nerve endings | Free nerve endings |
| Afferents | Aδ (group III) fibres | C (group IV) fibres |
| Action potential conduction velocity | Relatively slow, 5-30 m/s | Very slow; 0.5-2 m/s |
| Subjective sensation | Sharp, pricking pain | Dull, burning, throbbing pain |
| Onset of sensation | Short latency, quick onset | Long latency, slow onset |
| Localization | Well localized, easily identified | Poorly localized, diffuse |
| Duration of sensation | Short lasting | Long lasting |
| Subjective response | Reflex withdrawal, less difficult to endure, possible emotional involvement | Emotional and automatic response |
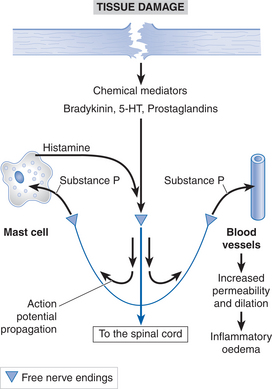
These two pain modalities underlie the concepts of transient and prolonged pain sensations. Transient pain is the first sensation to accompany a noxious stimulus and usually involves only minimal tissue damage. It is of short duration and has no real long-term consequences for the subject. The Aδ afferent nerve fibres that are responsible for these sensations are also involved in the withdrawal reflex (see below). Prolonged pain is associated with activation of the group C afferent nerve fibres and usually accompanies a greater degree of tissue damage. This damage to the tissue cells results in the release of chemical mediators — such as bradykinin, substance P, histamine, 5-hydroxytryptamine (5-HT) and prostaglandins — from the damaged cells themselves and from activated nociceptor nerve endings. These chemical mediators can activate nociceptive nerve terminals directly and can also sensitize the response of the nociceptors to normal stimuli by altering the transduction properties of the free nerve endings. As well as activating group C nociceptive endings, these chemical mediators are also responsible for initiating the inflammatory responses in the damaged tissue. Figure 6.2 summarizes how tissue damage and the release of chemical mediators can activate nociceptors and transmit this information to the CNS.
CENTRAL ASPECTS
Information from the nociceptive afferent nerves is transmitted to the spinal cord, where it influences reflex activity, or is further transmitted via specific pathways to higher brain centres. Nociceptive afferents enter the spinal cord via the dorsal root and make synaptic connections with other neurons located in the dorsal horn of the spinal cord grey matter. The dorsal horn is the site of convergence of several inputs relating to nociception, including the peripheral afferents described above, spinal interneurons and also descending neurons from higher centres in the brain.
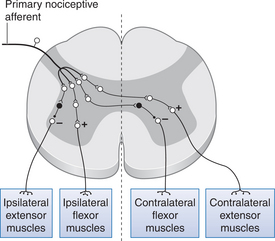
The main reflexes involving nociceptive afferents are the flexor withdrawal and crossed extensor reflexes. These are polysynaptic reflexes and involve several muscle groups as well as operating over several spinal segmental levels. Nociceptive inputs make excitatory polysynaptic connections with motoneurons supplying flexor muscle groups and inhibitory polysynaptic connections with extensor motoneurons on the ipsilateral side. When these pathways are activated, they produce flexion in the limb where the original noxious stimulus arose while simultaneously switching off activity in the extensor muscles of this limb. These actions serve to move the limb away from the initial stimulus and, therefore, act in a protective fashion by removing the area from potential damage. At the same time, different polysynaptic connections from the same nociceptive afferents excite extensor motoneurons and inhibit flexor motoneurons in the contralateral limb. This action serves to stabilize the body during flexion of the ipsilateral limb. Figure 6.3 summarizes these connections.
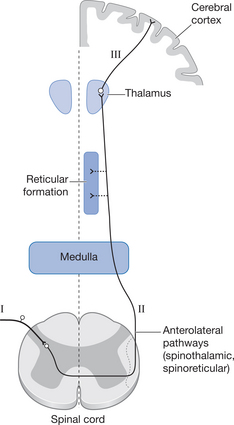
The nociceptive afferents entering the spinal cord grey matter terminate in the dorsal horn, where they make synaptic connections either with interneurons serving the reflexes described above or with second-order neurons (so-called transmission cells, or T cells). These cross the midline of the spinal cord to transmit information to the higher centres via the lateral spinothalamic pathways on the contralateral side of the spinal cord (Fig. 6.4). The axons travelling in these pathways are therefore always second-order neurons, which have their cell bodies in the marginal zone or substantia gelatinosa (SG) of the spinal cord grey matter. Some of these second-order axons will ascend ipsilaterally for a few spinal segments before crossing the midline, whereas others will cross immediately. When these ascending neurons reach the ventrobasal nucleus of the thalamus, they terminate on third-order neurons, which then convey the information on the noxious stimulus to the cerebral cortex.
MODULATION OF PAIN TRANSMISSION
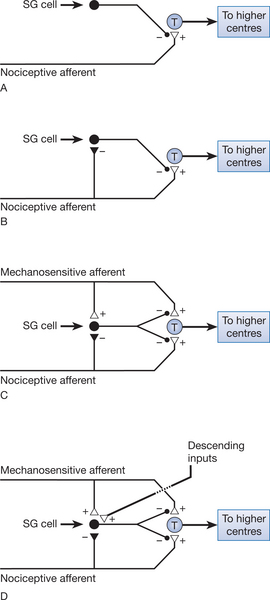
As we have already noted, primary nociceptive afferents terminate on second-order neurons, which then transmit the nociceptive information to higher centres. The excitability of this pathway can be altered by other interneurons present in the dorsal horn. Cells of the substantia gelatinosa (SG cells) have an inhibitory influence on the transmission cells. This is achieved by presynaptic inhibition of the nociceptive afferent terminals at the point where they synapse with the transmission cells (Fig. 6.5A). However, the SG cells are inhibited when the nociceptive afferents are activated (Fig. 6.5B), reducing the presynaptic inhibition of the nociceptor afferent terminal and thereby allowing nociceptive information to be passed to higher centres.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree