Fig. 1
Brain water content (a), tumor wet weight (b), and tumor dry weight (c) in the brains of WT and KO mice at day 12 after F98 cells were implanted into the right caudate. Values are mean ± SD, n = 8, *p < 0.05 by Student’s t-test
Protein levels of HIF-1α in the ipsilateral hemisphere were higher in WT mice (373 ± 25 pg/g brain tissue) than in KO mice (333 ± 35 pg/g brain tissue, p < 0.05) at 7 days after F98 cells implantation. VEGF protein levels were also higher in the ipsilateral hemisphere of WT mice (219 ± 21 vs 166 ± 22 pg/g brain tissue in KO mice, p < 0.01).
On T2-weighted images, the tumor showed hypointensity and isointensity compared with brain tissue, whereas peritumoral tissue was hyperintense. The brain tissue around tumor was compressed and the midline was shifted. Tumor size in WT mice was bigger than in KO mice on both T2 and T1 images. On T1-weighted images, after IP injection of Magnevist, the tumor was more obvious with its mass effect and peritumor edema. The tumor volume measured in T1 ivmages was larger in WT mice (44.0 ± 13.0 mm3) than in KO mice (16.9 ± 12.5 mm3, p < 0.05, Fig. 2a) at day 7. Because the boundaries of the tumors were clearly demarcated, we measured the total area of tumor, which appears bigger than H&E tumor staining after tissue processing. However, H&E-stained sections also demonstrated larger tumor volumes in WT than in KO mice at day 12 (WT: 23.2 ± 12.6 mm3, PAR-1 KO mice: 8.6 ± 9.7 mm3, p < 0.05; Fig. 2b). Tumors could be seen extending to the brain surface in WT mice but not in KO mice (Fig. 2b).
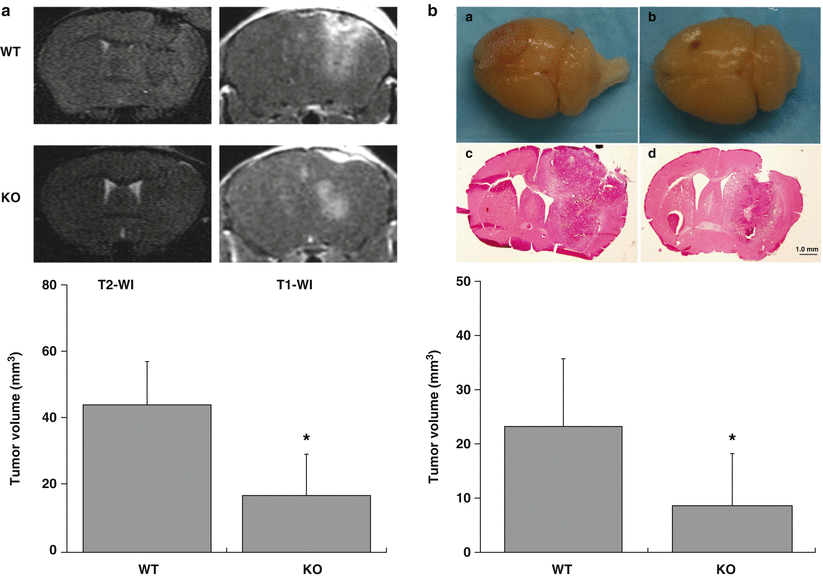

Fig. 2
(a) T2- and T1-weighted images and the tumor volume measured in T1 images at 7 days. (b) Pictures of the brain and tumor in H&E-stained sections in WT (a, c) or PAR-1 KO (b, d) mice at day 12 after F98 cells implantation. The bar graph shows quantification of tumor volumes. Values are mean ± SD, n = 7–9, *p < 0.05 by Student’s t-test
Discussion
In this study, we found that F98 glioma cell implantation caused lower brain edema, less tumor mass and volume, and lower HIF-1α and VEGF expression levels in PAR-1 knockout compared with WT mice.
Thrombin is a key factor in the common pathway for activation of the coagulation system [10, 32] and may act as a growth factor for tumor cells [3]. Malignant tumors express high levels of active tissue factor, an element of the extrinsic coagulation cascade that ultimately converts prothrombin to thrombin [22, 23, 25]. In our previous studies, thrombin was detected in gliomas, and the thrombin inhibitor argatroban attenuated brain edema, tumor mass, neurological deficits, and prolonged survival time [11, 13, 14].
The tumor microenvironment is important in tumor growth and invasion [23]. Prothrombin and PAR-1 are widely distributed throughout the brain tissue (e.g., astrocytes, microglia, and neurons) [17, 29]. PAR-1 deficiency provides an opportunity to explore the role of this receptor in thrombin signaling [4] in relation to tumor biology. In the current study, PAR-1 deletion in the mouse host reduced HIF-1α and VEGF expression levels after F98 cell implantation. These two changes may be linked as VEGF is regulated by HIF-1α, which also regulates the expression of many other genes involved in the response to hypoxic environments [30]. We have previously shown that thrombin upregulates HIF-1α in brain [16] and our current results suggest that this is at least in part via PAR-1, as also suggested for colorectal cancer cells [1].
VEGF has been recognized as a major angiogenic factor [24, 31]. Angiogenesis is the process of generating new blood vessels from preexisting vessels and is considered essential for tumor growth and metastasis. The presence of vascular endothelial proliferation is significantly associated with malignancy and contributes to the extent of peritumoral edema. Thus, the decrease in VEGF levels in the KO mouse may inhibit angiogenesis and contribute to the observed reduction in tumor growth. Increased thrombin signaling, by treating glioma cells with thrombin, increased tumor growth induced by thrombin treated tumor cells [12].
Thrombin signaling may impact angiogenesis via multiple factors. Thus, thrombin also increases the expression and release of angiopoietin-2 [15] and decreases adhesion of endothelial cells to basement membrane proteins, thus facilitating their detachment and migration to distal sites and vascular remodeling [26, 27].
The current study used PAR-1 KO mice but the injected glioma cells may still express PAR-1. Thus, PAR-1 antagonists may be of benefit in glioma by downregulating VEGF production by both the tumor and peritumoral tissue.
In conclusion, the thrombin receptor PAR-1 may play an important role in glioma growth. This provides us a new strategy to treat gliomas in humans and needs further investigation.
Acknowledgment
This study was supported by grants NS-073595, NS-079157, and NS-084049 from the National Institutes of Health (NIH) and NSFC30901549 from National Science Foundation of China.
References
1.
2.
Cheng Y, Xi G, Jin H, Keep RF, Feng J, Hua Y (2014) Thrombin-induced cerebral hemorrhage: role of protease-activated receptor-1. Transl Stroke Res 5:472–475PubMedCentralCrossRefPubMed
3.
Chinni C, de Niese MR, Tew DJ, Jenkins AL, Bottomley SP, Mackie EJ (1999) Thrombin, a survival factor for cultured myoblasts. J Biol Chem 274:9169–9174CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








