Fig. 1
Effects of imatinib treatment after SAH on EBI in the left frontal cortex at bregma + 1 mm. Representative SAH brain slice showing the left frontal cortex (a). Representative images showing TUNEL-positive neurons (arrows) (b) and quantitative analysis of TUNEL-positive neurons (c). Sham-PBS sham-operated rats treated with PBS, SAH-PBS SAH-imatinib, SAH rats treated with PBS or imatinib, data mean ± SEM, P value, ANOVA. N = 4 per group. Bar = 50 μm
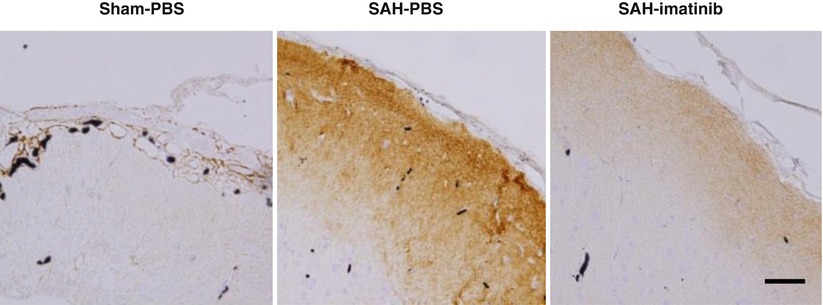
Fig. 2
Effects of imatinib treatment after SAH on immunohistochemical TNC staining in the left frontal cortex at bregma + 1 mm at 24 h after SAH. Sham-PBS sham-operated rats treated with PBS, SAH-PBS or SAH-imatinib SAH rats treated with PBS or imatinib, Bar = 50 μm
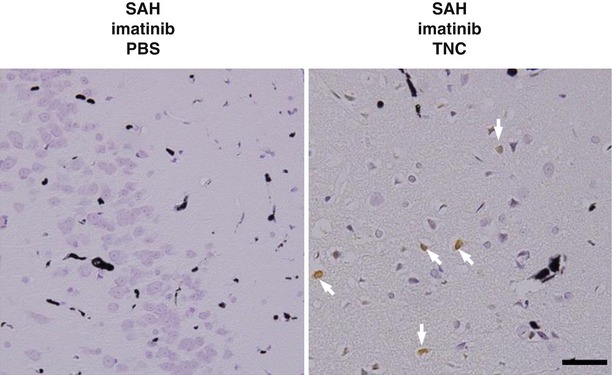
Fig. 3
Effects of intracisternal infusions of recombinant TNC on EBI in SAH rats treated with imatinib (SAH-imatinib) at 24 h after SAH. SAH-imatinib-PBS or SAH-imatinib-TNC, SAH-imatinib with a pre-SAH intracisternal infusion of PBS or TNC. Arrows, TUNEL-positive neurons. Bar = 50 μm
Discussion
In our recent study, TNC induced cerebral vasospasm after SAH via activating p38 and upregulating PDGFR-β; and imatinib prevented cerebral vasospasm via inhibiting TNC expression [10]. This study also showed that SAH upregulated TNC in the cerebral cortex with induction of TUNEL-positive neurons, and inhibition of TNC by imatinib decreased the number of TUNEL-positive neurons. Recombinant TNC injections reincreased TUNEL-positive neurons in SAH brain regardless of imatinib treatment. These findings suggested that TNC was involved in the pathogenesis of EBI after SAH.
TNC, a matricellular protein, is highly expressed in embryonic tissue during morphogenesis and sparsely expressed in the adult, but reappears in pathological states [3]. It has been reported that TNC can activate mitogen-activated protein kinases and some growth factor receptors, and can upregulate proinflammatory cytokines [8] and endothelin receptor type A [6]. By these means, TNC has multiple functions in the regulation of cell migration, proliferation, and apoptosis [5]. Apoptosis, inflammation, and endothelin-1 release play an important role in the mechanisms of EBI after SAH [9]. Thus, TNC may have a significant role in the pathophysiology of EBI after SAH, but the mechanisms to induce EBI remain unclear and need further studies to clarify the mechanisms.
After SAH, platelets activate and aggregate in the lumen of small cerebral vessels [9]. Luminal platelet aggregates activate and promote mechanisms that cause structural injury and functional deficits in small vessels, and devastate the already compromised brain. PDGF can induce TNC expression via the phosphoinositide 3-kinase/Akt pathway [4] and MAPK pathway [1]. In addition, our previous study suggested that PDGF-induced TNC may positively feedback on PDGFR activation via PDGFR upregulation and crosstalk signaling between receptors as well as upregulation of TNC itself, leading to MAPK activation and cerebral vasospasm after SAH [10]. Thus, PDGF-induced TNC signaling is suggested to be involved in the induction of EBI after SAH.
Recent research efforts moved to focus on clarifying the pathophysiology of EBI after SAH and on developing protective strategies against EBI to improve outcome after SAH [9]. Neuronal apoptosis is involved in the pathogenesis of EBI after SAH [2, 9]. This study showed that TNC may induce neuronal apoptosis, but further studies are needed to prove the underlying mechanisms.
Conclusion
TNC may play an important role in the pathogenesis of EBI after SAH. Further investigations are necessary to understand the mechanism behind how TNC affects EBI and to determine a new therapeutic approach against EBI for improving neurological outcome after SAH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






