For example, if we take a patient with an AHI of 38 (AHIoffCPAP). Using the compliance criteria cut-off discussed, our patient sleeps using CPAP 7 nights per week (NIGHTSonCPAP). The AHI (AHIoffCPAP) is reduced to 2 (AHIonCPAP) during 4 h, again using the compliance cut-off criteria discussed (HOURSonCPAP). During the residual 4 h (HOURSoffCPAP), the AHI remains 38. Using the generalized formula above and the parameters for this patient, we can calculate the mean AHI during compliant use of CPAP:
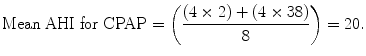
This formula can be generalized to other PSG outcomes such as the apnea index or desaturation index. This mathematical formula is based on the assumption that the AHI will revert to baseline once the CPAP appliance is no longer used. CPAP is thought to play a role in reducing edema resulting from snoring-associated vibration and apnea-induced mechanical stress of the upper airway. It can be argued that the baseline AHI may be reduced by a fraction in chronic CPAP use and that after termination of CPAP during the night the AHI may not completely revert to baseline. The precise effect however remains to be elucidated. If future research allows quantification of the magnitude of this effect, the formula could easily be extended by a factor addressing this aspect.
Future Perspectives
Treatment outcome based on individual compliance in conservative treatment can currently most reliably be reported in patients with CPAP. Built-in counters have become a standard feature in CPAP devices, and hours of use can easily be assessed by every physician. Until recently accurate assessment of compliance for other conservative interventions was limited to subjective self-report.
Reporting on the efficacy of OA in a 3-month prospective clinical trial, Vanderveken et al. took objective OA compliance into consideration through an embedded microsensor thermometer with on-chip integrated readout electronics [52]. The mean AHI was calculated based on the objective OA use and treatment period. Their results support the hypothesis that higher compliance with OA therapy translates into a similar adjusted effectiveness as compared with CPAP [52, 67]. Despite not being a common practice as yet, compliance to OA devices can be measured objectively with the introduction of this new device [52].
In future studies comparing the effects of different devices (e.g. CPAP or OA) on the AHI with alternative treatment methods, especially those with 100 % adherence (e.g. surgery), adherence should to be taken into account with the formula mentioned above. In doing so, one could compare the effectiveness of OA, CPAP and surgery.
The following example may illustrate this approach: in a recent systematic review and meta-analysis reporting on the efficacy of maxillomandibular advancement (MMA) on the AHI in OSA patients, the mean AHI decreased from 63.9 to 9.5 per hour (p < 0.001) following surgery [12].
In the previously mentioned study by Stuck et al. addressing the effects of CPAP, the mean AHI decreased from 35.6 to 11.9 per hour when individual adherence was taken into account [12]. The mean AHI under CPAP was 2.4 per hour. Juxtaposed, these treatment modalities seem to be equally effective in reducing the AHI when adherence is taken into account, although the population in the MMA study was more severely affected. This approach may also be used to compare the effects of other current treatment strategies.
References
1.
Strohl KP. Overview of obstructive sleep apnoea in adults. In: Basner RC, editor. UpToDate 20S12.
2.
Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5.
3.
Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;CD001106.
4.
McNicholas WT, Bonsignore MR, editors. Sleep apnoea. European Respiratory Society Monograph; 2010.
5.
Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401.PubMed
6.
Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;CD004435.
7.
Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnoea: a review. Sleep. 2006;29:244–62.PubMed
8.
9.
10.
11.
12.
Johnston CD, Gleadhill IC, Cinnamond MJ, Gabbey J, Burden DJ. Mandibular advancement appliances and obstructive sleep apnoea: a randomized clinical trial. Eur J Orthod. 2002;24:251–62.
13.
14.
Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9.
15.
16.
Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Eur J Orthod. 2002;24:239–49.
17.
Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52:362–8.
18.
Tegelberg A, Wilhelmsson B, Walker-Engstrom ML, et al. Effects and adverse events of a dental appliance for treatment of obstructive sleep apnoea. Swed Dent J. 1999;23:117–26.
19.
20.
Walker-Engstrom ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A. A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnoea. Sleep Breath. 2003;7:119–30.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







