Overview and Perspective
At the time of the first edition of this book, there existed only one double-blind, placebo-controlled trial of omega-3 fatty acids in bipolar disorder, and no controlled studies in unipolar major depression were yet published. Now, a mere 4 years later, the number of double-blind, placebo-controlled trials stand at 4 for bipolar disorder and 7 for unipolar major depression. Most, but not all of these clinical trials have reported that the omega-3 fatty acids were superior to placebo. These somewhat mixed results could perhaps have been expected from such a collection of heterogeneous and small studies, which investigated a complex group of multifactorial psychiatric disorders. This chapter provides an up-todate review of the basic and clinical research findings with regard to omega-3 fatty acids and mood disorders and attempt to reconcile the discrepant clinical trial findings.
Origin and Chemistry of the Omega-3 Fatty Acids
The omega-3 fatty acids, along with their counterparts, the omega-6 fatty acids, are a group of crucial naturally occurring lipids, which collectively are termed essential polyunsaturated fatty acids (PUFA) (
1,
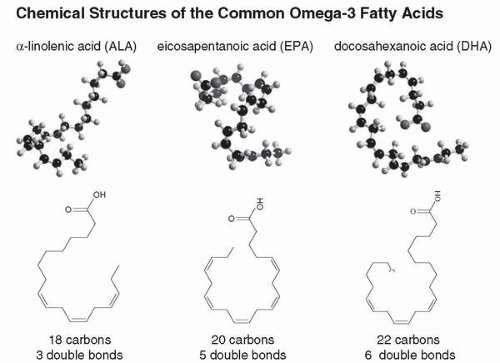
2). The three predominant, naturally occurring omega-3 fatty acids are as follows: docosahexanoic acid (DHA), eicosapentanoic acid (EPA), and α-linolenic acid (ALA). The first two (DHA and EPA) are known as the long-chain omega-3 fatty acids, and are found primarily in fish oil and other marine sources (
3). The shorter-chain ALA is an omega-3 fatty acid obtained from certain species of terrestrial plants (e.g., flaxseed, purslane, and others) (
4,
5). The chemical structures of the omega-3 fatty acids are shown in
Figure 4.1.
Omega-6 fatty acids, such as the 18-carbon linoleic acid (LA) and the 20-carbon arachidonic acid (AA), are derived primarily from vegetable oils and are ubiquitous in the food supply of Western countries (
1,
6,
7). AA and EPA are nearly identical chemically (differing only by the presence of the double bond in the 3-position in EPA and the consequent 2 additional hydrogen atoms in AA), yet have opposing actions on inflammatory processes in the body. Vertebrate animal species cannot desaturate (or add a double bond to) fatty acids before the ninth carbon atom from the lipophilic end of the carbon chain (
8). Thus, the omega-3, the omega-6, and the omega-9 classes of fatty acids, with double bonds beginning at the third, sixth, and ninth carbon atoms, respectively, must be obtained from the diet (hence the term “essential”). The origin of the long-chain omega-3 fatty acids is photosynthesis within the chloroplasts of marine phytoplankton (
9). The EPA and DHA are then passed through the food web, and ultimately to humans.
The major differences among the various omega-3 fatty acids are the length of the carbon chain and the number of double bonds (
Fig. 4.1). DHA has a 22-carbon chain with six double bonds, EPA has a 20-carbon chain with five double bonds, and ALA has 18-carbon chain with three double bonds (
1). In the omega-3 fatty acids, the double bonds recur every third carbon atom, and
in vivo, humans can theoretically convert one omega-3 fatty acid into another. However, the rate-limiting step in the conversion process involves the enzyme Δ-6 desaturase (
10), and the process of elongation is limited (
10). Although controversial, the most recent data indicate that most people cannot adequately convert ALA into EPA and DHA to meet their nutritional needs (
11,
12,
13). In one recent human study, less than 8% of ALA was converted into EPA and less than 0.5% of ALA was converted into DHA (
12). Women convert more ALA into DHA than men, due to an estrogen-dependent up-regulation of hepatic DHA synthesis from EPA and ALA, particularly during pregnancy (
14,
15). Thus, it appears to be crucial for men to obtain EPA and DHA directly from marine or other sources. For women, it is not clear if the estrogen-mediated increase in DHA is adequate to meet the nutritional need for EPA and DHA, especially when not pregnant.