One-Carbon Metabolism and the Treatment of Depression: Roles of S-Adenosyl-L-Methionine and Folate
Jonathan E. Alpert
George I. Papakostas
David Mischoulon
The putative psychotropics reviewed in the chapter are formulations of the B-vitamin folic acid as well as S-adenosyl-L-methionine (SAMe), a cofactor in various metabolic processes in the brain and elsewhere in the body. These substances are discussed together based on their interrelationship in the metabolic pathways referred to as the one-carbon cycle. Beginning with the observation over 40 years ago that depressive symptoms could be related to selective deficiencies of folate, studies have continued to accrue that demonstrate some form of relationship between depression and folate deficiency in community and clinical samples. Similarly, a number of small-to-moderate sized controlled treatment trials among depressed adults have suggested potential antidepressant efficacy for various forms of folate as well as oral and parenteral forms of SAMe. These studies have continued to fuel interest in the one-carbon cycle as a potential source of insights about the pathophysiology, prevention, and optimal treatment of depressive disorders.
S-ADENOSYL-L-METHIONINE
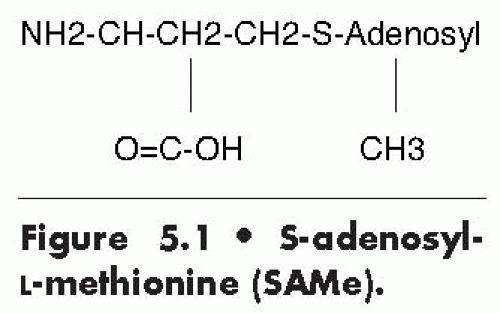
SAMe is a naturally occurring substance discovered in 1952 that is produced in mammals from L-methionine and adenosine triphosphate (Fig. 5.1). SAMe has been studied in Europe for over 35 years as a treatment for depression and other disorders. However, in the United States, SAMe has risen from relative obscurity only following its release in 1999 as an over-the-counter dietary supplement. With claims to promote emotional well-being as well as joint health, SAMe has been the subject of national media coverage and several popular books. However, its use has been somewhat limited, in part due to its relatively high cost compared with other over-the-counter supplements and due to the paucity of data on stable, oral forms of SAMe compared with contemporary first-line antidepressants.
Unlike St. John’s wort, SAMe is not a plant extract, a vitamin, or a mineral. However, SAMe is ubiquitous among living organisms, and it plays a critical role in a broad range of metabolic reactions. It is found throughout the human body, although particularly high concentrations have been measured in the liver, adrenal glands, and the pineal gland. SAMe appears to be uniformly distributed in the brain where it serves as a major donor of the methyl groups required for key transmethylation reactions.
SAMe is involved in the synthesis of a large number of neural messengers, including the monoamines (e.g., norepinephrine, dopamine, and serotonin) and melatonin, and of neuronal membrane constituents, particularly phospholipids, which play a critical role in signal transduction. SAMe has been traditionally thought to function in the central nervous system (CNS) mainly through methyl-transferase reactions that shift the methyl group of SAMe to
a wide variety of methyl acceptor molecules, such as catecholamines and other biogenic amines, proteins, and phospholipids (1).
a wide variety of methyl acceptor molecules, such as catecholamines and other biogenic amines, proteins, and phospholipids (1).
Nevertheless, SAMe also takes part in other metabolic pathways, including (a) transsulfuration; (b) aminopropylation; (c) synthesis of proteoglycans for cartilage; (d) formation of cysteine and glutathione and other endogenous anti-inflammatory, neurotrophic, and antioxidant substances; and (e) synthesis, repair, and recombination of DNA (2). In view of its widespread distribution and pervasive metabolic functions, it is not surprising that SAMe has been advanced—with varying degrees of empirical support—as a treatment for a broad range of conditions in addition to clinical depression; these include dementia, Parkinson disease, cholestasis, osteoarthritis, and fibromyalgia (3,4).
SAMe is continuously recycled in the body within the one-carbon cycle. It is formed from the essential amino acid L-methionine in a reaction that requires adequate amounts of vitamin B12 and folic acid. Since dietary sources of L-methionine are insufficient, SAMe synthesis also depends on the endogenous formation of this amino acid from homocysteine. SAMe, in turn, is ultimately metabolized back to homocysteine, thereby replenishing the substrate needed to synthesize L-methionine and, eventually, to produce SAMe once again.
S-Adenosyl-L-Methionine and Methylation in Depression
Smythies (5) formulated the one-carbon cycle hypothesis that a defect in the mechanism of the one-carbon cycle causes some psychiatric illnesses. Tolbert et al. (6) observed that patients with unipolar depression had significantly lower levels of methionine adenosine transferase (MAT), which is one of the enzymes of the one-carbon cycle (Fig. 5.2). Smythies et al. (7) also reported that, following antidepressant treatment, patients with major depression displayed a significant increase in the Vmax (the highest amount of substrate per unit time that an enzyme can process at saturation) of their MAT activity. L-Methionine is converted by MAT into SAMe, which, in turn, donates its methyl group to a variety of molecules in the brain (8). Reynolds et al. (9) hypothesized that a methylation deficit occurred in depression, based primarily on the discovery of a reduction in CNS methylation in depression that was reversed with recovery. In support of this view, cerebrospinal fluid (CSF) SAMe levels were found to be significantly lower in severely depressed patients than in a neurologic control group (10).
Also of potential interest is the effect of SAMe on neuronal membranes, since it promotes the conversion of phosphatidylethanolamine into phosphatidylcholine, thereby increasing the fluidity of cell membranes and possibly facilitating neurotransmission by increasing the density of available receptors or the efficiency of receptor-effector coupling (11). Small alterations in membrane molecular dynamics can produce significant effects on signal transduction across cell membranes (12,13); in patients with dementia, the intravenous (IV) administration of SAMe has been reported to decrease the microviscosity of platelet membranes (14). In a study of patients with depression (15), the overall effects of oral SAMe on membrane fluidity were quite inconsistent. Nine depressed patients showed a fairly significant increase in platelet membrane fluidity after treatment with SAMe; but the membrane fluidity actually decreased in seven patients, and it was essentially unchanged in three.
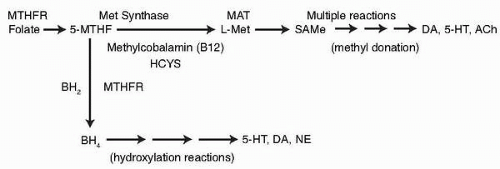 Abbreviations: 5HT, serotonin (5-hydroxy tryptophan); Ach, acetyl choline; BH2, quinonoid dihydrobiopterin; BH4, tetrahydrobiopterin; DA, dopamine; HCYS, homocysteine; L-Met, methionine; MAT, methionine adenosine transferase; Met Synthase, methionine synthase; MTHF, methyltetrahydrofolate; MTHFR, methylenetetrahydrofolate reductase; NE, norepinephrine. (Data from Mischoulon D, Raab MF. The role of folate in depression and dementia. J Clin Psychiatry 2007;68(10)(suppl):28-33 and Bottiglieri T, Hyland K, Laundy M, Godfrey P, Carney MW, Toone BK, Reynolds EH. Folate deficiency, biopterin and monoamine metabolism in depression. Psychol Med 1992;22:871-876, used with permission.) |
Although the effects of SAMe on monoamines are not consistent, SAMe does have an impact on monoaminergic systems (1,2). SAMe is involved in the methylation of catecholamines, and it appears to increase the serotonin turnover (16,17), to inhibit the reuptake of norepinephrine in a temperature-dependent fashion (11), and to augment dopaminergic activity (16). Increased CSF levels of homovanillic acid (HVA) have also been correlated with increasing CSF levels of SAMe (18). Reports of decreased prolactin secretion (17,19) may also be indirect evidence of an increased dopaminergic transmission. More recently, in the context of newer hypotheses about the mechanism of action of antidepressants, SAMe’s impact has been studied on brain neutrophic activity (20), inflammatory cytokines (21), and bioenergetics (22), which suggest additional routes through which SAMe may exert a therapeutic effect on depression.
Clinical Studies with S-Adenosyl-L-Methionine in Depression
The first clinical trial of SAMe in the treatment of depression was published in 1973 (23). Since then, nearly 40 controlled and uncontrolled clinical trials have been reported. Most, though not all, have shown some degree of antidepressant benefit from oral, intravenous, or intramuscular administration of the compound.
After an initial, serendipitous observation of mood elevation in patients treated with SAMe (24), Fazio et al. (23) reported remission in 14 of 35 depressed patients in an open trial of parenteral SAMe. In 1975, Agnoli et al. (25) reported marked improvement of depression in 30 of 51 patients who were given intramuscular SAMe. Following these two reports, several open studies (26, 27, 28) showed that treatment with parenteral SAMe was followed by a marked improvement in a substantial proportion of patients. Lipinski et al. (29), using IV SAMe in a single-blind study of depressed inpatients, reported improvement or remission in seven of nine subjects. The antidepressant response was rapid and without side effects. Two other uncontrolled trials (30,31) suggested the efficacy of SAMe in depression.
Several double-blind studies showed that parenteral SAMe, when compared with a number of standard tricyclic antidepressants (TCAs), such as clomipramine, amitriptyline, and
imipramine, was generally equally or more effective; and it tended to produce an earlier response (often within 3 to 7 days) and fewer side effects (32, 33, 34, 35, 36, 37, 38, 39, 40). Similarly, in studies examining the efficacy of IV SAMe compared with a placebo, the SAMe was significantly more effective (26,40), demonstrating a 56% response rate compared to a 13% response rate with placebo.
imipramine, was generally equally or more effective; and it tended to produce an earlier response (often within 3 to 7 days) and fewer side effects (32, 33, 34, 35, 36, 37, 38, 39, 40). Similarly, in studies examining the efficacy of IV SAMe compared with a placebo, the SAMe was significantly more effective (26,40), demonstrating a 56% response rate compared to a 13% response rate with placebo.
Although parenteral SAMe appears to be an effective antidepressant, whether similar clinical efficacy can be achieved with its oral preparation is unclear. A highly labile compound, SAMe may be too labile and too polar to survive absorption without giving up its methyl group. On the other hand, the oral administration of SAMe is associated with a significant rise of CSF SAMe, suggesting that it crosses the blood-brain barrier in humans (10). In an open trial conducted with oral SAMe on 11 nontreatment-resistant depressed outpatients and nine treatment-resistant depressed outpatients (15), eight (73%) of the 11 nontreatment-resistant patients were considered responders. Complete response was defined as a greater than 50% reduction in the 21-item Hamilton Depression (HAMD) rating scale (41) score from baseline, an end point Clinician Global Impression Severity Scale (CGI-S) (42) score of 1 or 2, and/or a CGI-S decrease greater than 2 points from baseline. The markedly significant clinical improvement found in this study following treatment with oral SAMe was also accompanied by significant neuroendocrine effects (19).
These findings, which suggest efficacy of the oral SAMe preparation, were confirmed by the double-blind studies by Kagan et al. (43) and Salmaggi et al. (44) showing that SAMe, 1,600 mg per day, was more effective than a placebo in treating depressive symptoms among inpatients with major depression (N= 18, where N is the number of patients) and depressed postmenopausal women (N=80). Bell et al. (45) and De Vanna and Rigamonti (46) also showed the efficacy of oral SAMe (1,600 mg per day) to be comparable to that of desipramine and imipramine in two depressed populations (N=28 and 30, respectively).
However, when Fava et al. (47) evaluated the efficacy of oral SAMe in a double-blind, placebo-controlled study of 44 depressed outpatients, the sample as a whole improved significantly during the administration of either oral SAMe or placebo, but a significant difference between treatments was not observed. In this study, the failure of SAMe to produce effects significantly different from those of placebo might have been due to problems in the stability of the new preparation of this compound; an analysis of the content of the 400-mg tablets of SAMe by Trapp et al. (unpublished results) suggested greater than 50% degradation of SAMe. One of the inferences that can be drawn from this experience is that low doses of SAMe may have little or no discernible antidepressant benefit over a placebo.
In a 1994 formal meta-analysis by Bressa (48), 13 prospective double-blind, randomized clinical trials were selected as having met the criteria for a meaningful comparison. In six of these studies, SAMe had been compared with a placebo. In the seven remaining studies, SAMe had been compared with one of the various TCAs. SAMe was found to be significantly more effective than the placebo and equivalently effective to and typically better tolerated than the TCAs in the treatment of depression. The meta-analysis (48) of all the studies with oral and parenteral SAMe (including the authors’) showed a superior response rate with SAMe when compared with the placebo, with an average global effect size of 27.5%. This effect size range is comparable to the average effect size of 25% for trazodone and two heterocyclic antidepressants (amoxapine and maprotiline) and is slightly higher than the average effect size of 19% for two standard TCAs (imipramine or amitriptyline) that was reported by Greenberg et al. (49) in their meta-analysis of 22 studies of antidepressant outcome. Further support for the comparable efficacy of SAMe when compared to TCAs is derived from the Bressa meta-analysis of all the trials comparing SAMe with other antidepressant agents (48). His analysis showed, among full responders, a global response of 61% for SAMe and of 59% for TCAs.
In a 2002 meta-analysis commissioned by the Agency for Healthcare Research, Hardy et al. (3) reported an effect size equivalent to a moderate (6 point; 95% confidence interval [CI] = 2.2 to 9.0; N=422) improvement on the Hamilton Depression (HAMD) rating scale at 3 weeks in all evaluable studies of parenteral and enteral SAMe versus placebo as monotherapy for depression. In the same monograph, there was no significant difference between SAMe and conventional (tricyclic) antidepressants on the HAMD scale, with an effect size of 0.08 (95% CI = − 0.17 to 0.32; N=1,015) in all evaluable studies in which SAMe was compared with an antidepressant comparator. The monograph by Hardy et al. (3) also analyzed studies of SAMe’s efficacy in other conditions, particularly osteoarthritis and cholestasis and determined that the evidence supporting SAMe’s efficacy in depression was generally the most well-developed. Both meta-analyses, therefore, reinforced the conclusion that SAMe appears to have some antidepressant efficacy as monotherapy for depression, as did a more recent systematic review of 11 reports (50), which also pointed out some of the limitations of the studies including that many of the studies involved parenteral, and therefore less clinically feasible, formulations of SAMe, or older, and therefore more unstable, oral formulations. In addition, it was also recognized that the TCA doses with which SAMe was compared were generally low and that TCAs are no longer the first-line agents used for the treatment of depression.
Of relevance to SAMe’s putative antidepressant efficacy is the observation by Carney et al. (28) that three of 12 responders to IV SAMe underwent an early switch to mania or hypomania. Furthermore, in an open trial of oral SAMe, Carney reported that three of the first six patients treated with oral SAMe (500 to 1,600 mg daily for 14 to 42 days) experienced 1 to 3 days of euthymia, followed by hypomanic switches with symptoms including increased speech and activity, grandiose ideas, and, in one subject, increased libido (51). These samples included subjects with bipolar, as well as unipolar depression, which undoubtedly contributed to the higher switch rates.
Although parenteral SAMe appears to have antidepressant efficacy, its route of administration diminishes its feasibility. However, SAMe oral preparations with adequate bioavailability appear to be more acceptable with relatively few side effects. Information on the safety of oral SAMe administered for 6 weeks in the treatment of depression is available. Although no long-term safety data are available in depression, a 2-year study of the use of SAMe in hepatic cirrhosis found this compound to be safe and devoid of significant side effects (52). The encouraging results obtained in the course of four of five previously published double-blind preliminary studies support the hypothesis that this compound, even in its oral form, may have antidepressant effects.
If safe and effective, a stable oral formulation of SAMe would be an innovative contribution to the antidepressant pharmacopoeia for a number of reasons. SAMe does not appear to cause sexual dysfunction, a common complaint with conventional antidepressant drugs, particularly the selective serotonin reuptake inhibitors (SSRIs); nor is it known to cause weight gain, a side effect that may cause significant distress and compliance problems.
In addition, previous work has suggested the possibility that SAMe may be associated with a shorter latency of response than the conventional antidepressant agents, which typically require as much as 3 to 6 weeks before significant improvement in depression severity is observed (4). If so, SAMe, as monotherapy or, potentially, as an augmenting agent to other treatments, could benefit individuals with severe depression by reducing their period of functional impairment, risk of suicide, and length of stay for inpatient treatment.
Finally, in view of the prevalence of depression that is refractory to standard treatment, there continues to be much interest in the development of antidepressant agents that exploit potentially different pharmacologic mechanisms. Adequately sized double-blind studies of stable oral formulations of SAMe compared with current first-line pharmacotherapy (e.g.,
SSRIs) and placebo in depressed outpatients are clearly needed to guide clinical recommendations as are studies assessing the safety and efficacy of SAMe as an adjunct to contemporary antidepressants for responding to depressions that have proven refractory to existing treatments.
SSRIs) and placebo in depressed outpatients are clearly needed to guide clinical recommendations as are studies assessing the safety and efficacy of SAMe as an adjunct to contemporary antidepressants for responding to depressions that have proven refractory to existing treatments.
Recommendations in Clinical Practice
When assessing the relevance of the reviewed studies to clinical practice, one must bear in mind that, in most studies, SAMe was administered at doses that are extremely high in comparison with the doses currently recommended by distributors in the United States. The studies cited in the meta-analysis involved the administration of up to 1,600 mg per day of oral SAMe. Other previous and current randomized control trials of SAMe have used doses as high as 3,200 mg per day. By comparison, the suggested oral dose of SAMe recommended by distributors of the dietary supplement is generally 400 mg per day. Clinicians and patients must consider the likelihood that daily doses in excess of the 400 mg generally recommended in package inserts will be needed when treating clinical forms of depression, although 400 mg may be an acceptable starting dose.
At a cost of approximately $0.75 or more per 200-mg tablet, a daily dose of 1,600 mg, or 8 tablets per day, comparable to the doses studied in the European trials, could easily exceed $180.00 per month, a cost not typically covered by health insurance. What may prove to be a full therapeutic dose for most patients may therefore be difficult to swallow for many, both literally and figuratively, and the risk that patients will gravitate toward subtherapeutic doses is high.
Remembering that a majority of the controlled studies that have been reported to date have involved the administration of parenteral rather than oral preparations of SAMe is also important, since the former route may deliver more active ingredient per milligram of intake. In addition, these studies typically examined only short-term outcomes, and they enrolled patients with a variety of depressive disorders, including dysthymia and bipolar disorder, rather than those with major depression alone. The degree to which the studies that have been conducted on SAMe are relevant to particular clinical situations must be judged, therefore, in the context of potential differences in doses, route of administration, lengths of treatment, and the depressive disorder under treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree